Catalyst for organopolysiloxane as well as preparation method and application of catalyst
A technology of polysiloxane and catalyst, which is applied in organic chemistry, silicon organic compounds, chemical instruments and methods, etc., can solve the problems of difficult control of polymer end group structure, affecting product performance, etc., and achieve good industrial application prospects and performance Stable, low hydroxyl content effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
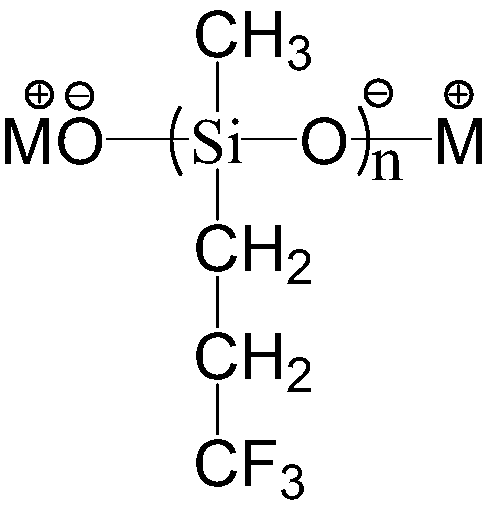
[0049] 1) Catalyst preparation: In a four-necked flask equipped with mechanical stirring, nitrogen, thermometer, and water separator, add potassium hydroxide aqueous solution (60wt%) 16.7g, trifluoropropylmethylcyclotrisiloxane 84g, benzene 60ml, heated to reflux under stirring, the solid gradually dissolved to form a uniform solution, and the generated water was removed by azeotropic water separator. After collecting 8.29g of water, it was reacted at constant temperature for 1 hour, and then the remaining benzene was evaporated at normal pressure, and then in Low volatile matter was extracted under reduced pressure at 60°C and 20mbar to obtain a colorless and transparent oil. The base value of the catalyst is obtained by acid-base titration, so that the n value can be converted, see Table 1 for details.
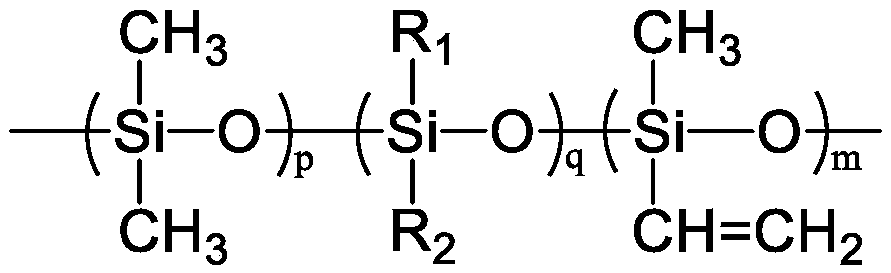
[0050] 2) Cyclosiloxane polymerization: After fully replacing the 2L planetary mixer with nitrogen, add 1000g of octamethylcyclotetrasiloxane to the kettle, dehydrate at 50°...
Embodiment 2
[0052] 1) Preparation of catalyst: In a four-necked flask equipped with mechanical stirring, nitrogen, thermometer, and water separator, add potassium hydroxide aqueous solution (60wt%) 16.7g, trifluoropropylmethylcyclotrisiloxane 84g, toluene 60ml, stirred and heated to reflux, the solid gradually dissolved to form a uniform solution, and the generated water was azeotropically removed by a water separator. After collecting 8.29g of water, it was reacted at a constant temperature for 1 hour, and then the remaining toluene was evaporated at normal pressure, and then in Low volatile matter was extracted under reduced pressure at 60°C and 20mbar to obtain a colorless and transparent oil.
[0053] 2) Cyclosiloxane polymerization: After the 2L planetary mixer was fully replaced by nitrogen, 1000g of octamethylcyclotetrasiloxane and 11.62g of tetramethyltetravinylcyclotetrasiloxane were added to the kettle, and the Dehydrate at 10mbar for 1h, then use nitrogen to break the air, add 41...
Embodiment 3
[0055] 1) Preparation of catalyst: In a four-necked flask equipped with mechanical stirring, nitrogen, thermometer, and water separator, 16.7 g of potassium hydroxide aqueous solution (60 wt %), 84 g of trifluoropropylmethylcyclotrisiloxane, and two With 60ml of toluene, heat up to reflux under stirring, the solid gradually dissolves to form a homogeneous solution, use a water separator to azeotropically remove the generated water, after collecting 8.29g of water, react at a constant temperature for 1 hour, then evaporate the remaining toluene under normal pressure, and then Low volatile matter was extracted under reduced pressure at 60°C and 20 mbar to obtain a colorless and transparent oil.
[0056] 2) Polymerization of cyclosiloxane: After fully replacing the 2L planetary mixer with nitrogen, add 1000g of octamethylcyclotetrasiloxane to the kettle, dehydrate at 50°C and 10mbar for 1 hour, then use nitrogen to break the air, and add 500 67.57g of vinyl-terminated polydimethy...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap