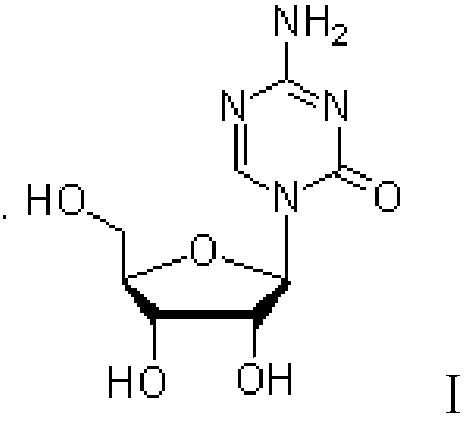
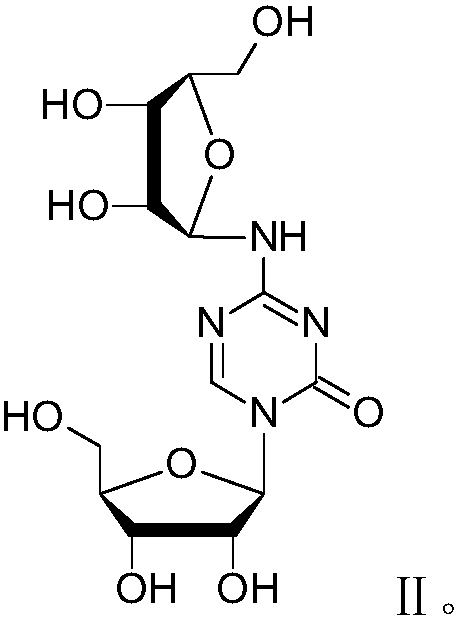
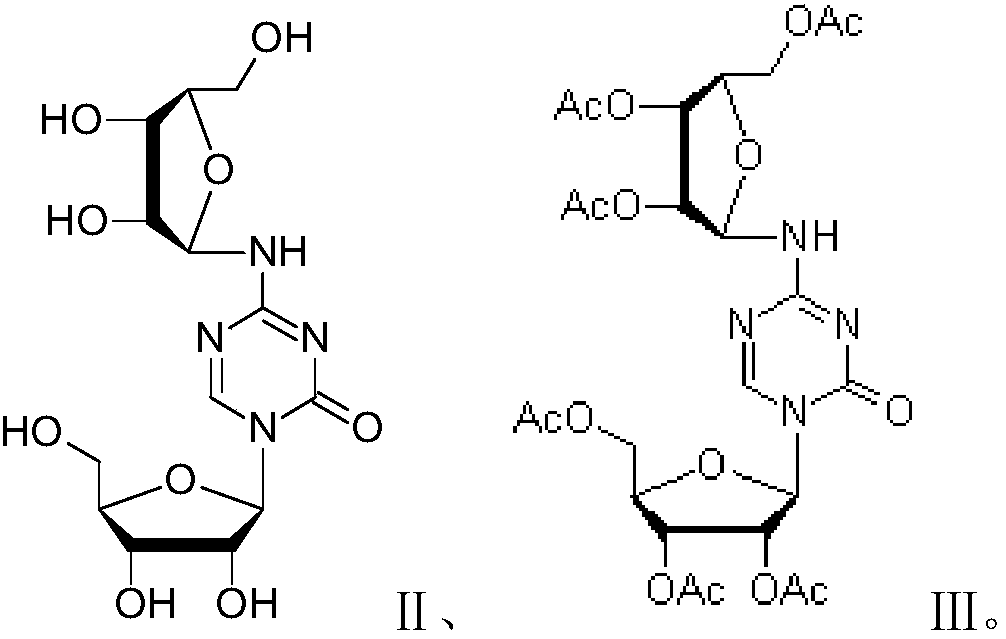
Azacitidine disaccharide impurity and preparation method and application thereof
A reaction, ribose technology, applied in the field of azacitidine disaccharide impurities and its preparation and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of formula III compound
[0029] Mix 20 g (62.9 mmol) of tetraacetyl ribose with 57 g (222.7 mmol) of the compound of formula IV, add dichloromethane, raise the temperature to 30°C, add 21 g (157.9 mmol) of aluminum trichloride, and react for 23 hours. Cool down to room temperature 25°C, pour the reaction solution into ice water of sodium bicarbonate, add dichloromethane at the same time, filter to remove insoluble matter, separate the layers, and concentrate the organic phase to dryness. Purified by silica gel column chromatography (eluent: dichloromethane / methanol=30:1) to obtain 24.1 g (38.3 mmol, yield 61%) of off-white solid.
Embodiment 2
[0030] Embodiment 2: the preparation of formula III compound
[0031] Mix 17g (53.4mmol) of tetraacetylribose with 55g (214.5mmol) of the compound of formula IV, add 1,2-dichloromethane, raise the temperature to 50°C, add 18g (133.5mmol) of aluminum trichloride, and react for 19 hours . Cool down to room temperature 25°C, pour the reaction solution into ice water of sodium bicarbonate, add dichloromethane at the same time, filter to remove insoluble matter, separate the layers, and concentrate the organic phase to dryness. Purified by silica gel column chromatography (eluent: dichloromethane / methanol=30:1) to obtain 19.1 g (30.4 mmol, yield 57%) of off-white solid.
Embodiment 3
[0032] Embodiment 3: the preparation of formula III compound
[0033] Mix 15g (47.1mmol) of tetraacetyl ribose with 60g (235.6mmol) of the compound of formula IV, add 1,2-dichloromethane, heat up to 30°C, add 42g (188.4 mmol.), reacted for 19 hours. Cool down to room temperature 25°C, pour the reaction solution into ice water of sodium bicarbonate, add dichloromethane at the same time, filter to remove insoluble matter, separate the layers, and concentrate the organic phase to dryness. Purified by silica gel column chromatography (eluent: dichloromethane / methanol=30:1) to obtain 13.0 g (20.7 mmol, yield 44%) of off-white solid.
[0034] The structure verification data of the compound represented by formula III are shown in Table 1 and Table 2.
[0035]
[0036] Table 1 Hydrogen Spectrum Determination Results
[0037] chemical shift
proton number
peak shape
Correlative proton chemical shift
attribution
Remark
9.18
1
d
5.70
H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com