Recombinant virus-like granule and preparation method and application thereof
A recombinant virus and virus-like technology, applied in the field of biomedicine, can solve problems such as hidden dangers, low-quality vaccines, and easily damaged particle assembly structures, and achieve the effect of efficient secondary assembly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0132] Example 1 Expression and Identification of Recombinant Hepatitis B Virus Core Antigen
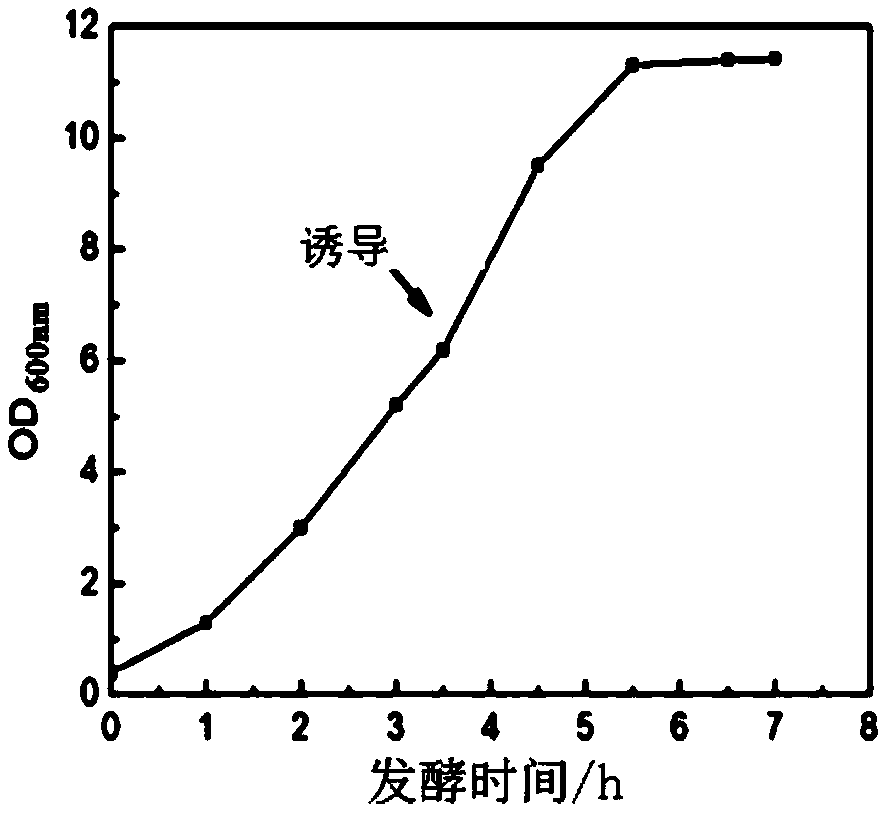
[0133] Whole Gene Synthetic HBc 149 The fragment was inserted into the vector pET28a through BamHI and HindIII sites, named pET28a-HBc 149 , subcloned into competent cells BL21(DE3), HBc 149 The amino acid sequence of the fragment is shown in SEQ ID NO.3. Inoculate to 20L fermenter for fermentation, wait for OD 600 To 5-8 o'clock, add 1mM IPTG induction 4h (thalline growth curve sees Figure 1A ), collected by centrifugation (induced expression identification results see Figure 1B ). Add the bacteriostasis solution (20mM Tris-HCl, 3mM EDTA, 0.5% Triton X-100, 1mM PMSF, pH 8.0) at a ratio of 1:10, and under the pressure of 800MPa, cyclically break through high-pressure homogenization three times, centrifuge Collect the supernatant, i.e. recombinant hepatitis B virus core antigen, transmission electron microscope (TEM) identification results see Figure 1C .
[0134] SEQ ID NO....
Embodiment 2
[0138] Example 2 Effects of Depolymerization Conditions on Protein Yield, Purification, Structure and VLP Extracellular Reassembly
[0139] 1. Effect of depolymerization conditions on protein yield
[0140] The crushed supernatant was precipitated with 1M ammonium sulfate, and denaturants of different types and concentrations were used to depolymerize the protein, and then the protein concentration was measured by the Bradford method (see Table 1 for the protein yield), and the depolymerized solution was identified by SDS-PAGE ( see results figure 2 ).
[0141] Table 1 Effect of different types and concentrations of denaturants on protein yield
[0142] Depolymerization conditions
Protein concentrationmg / ml
protein yield
2M urea
3.64
72.8%
4M urea
4.59
91.8%
6M urea
4.83
96.6%
8M urea
4.94
98.8%
6M GdnHCl
4.99
99.8%
[0143] From Table 1 and figure 2 It can be seen that when the concen...
Embodiment 3
[0152] Example 3 Effect of Extracellular Assembly Conditions on the Assembly of Virus-like Particles of HBc
[0153] The extracellular assembly of virus-like particles is mainly affected by temperature, pH value of solution, surfactant, reaction time and ionic strength, which were investigated in this experiment. Samples depolymerized with 4M urea were purified by Ni FF and dialyzed first into 2M urea and then into the respective assembly buffers.
[0154] The characterization method mainly adopts TSK G4000SWxl with a flow rate of 0.6ml / min. By corresponding with the TEM results, it is determined that the peak time is 9.5min for aggregates, 10.6min for assemblies and 19.4min for unassembled structures.
[0155] 1. The influence of reaction temperature on assembly: the reaction temperature mainly affects the reaction rate, such as Figure 7A As shown, the protein folding speed is too fast at room temperature, and most of the protein is precipitated, but at 4°C, the protein is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com