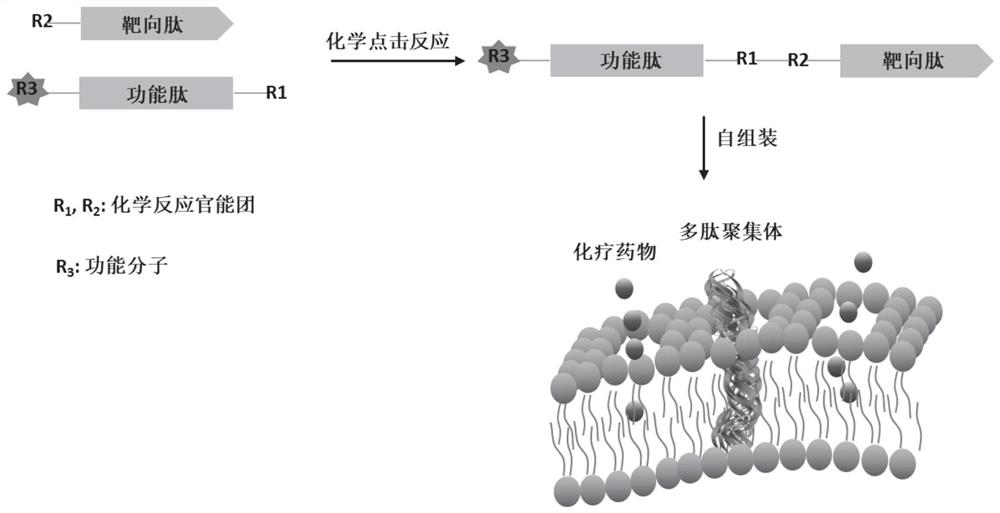
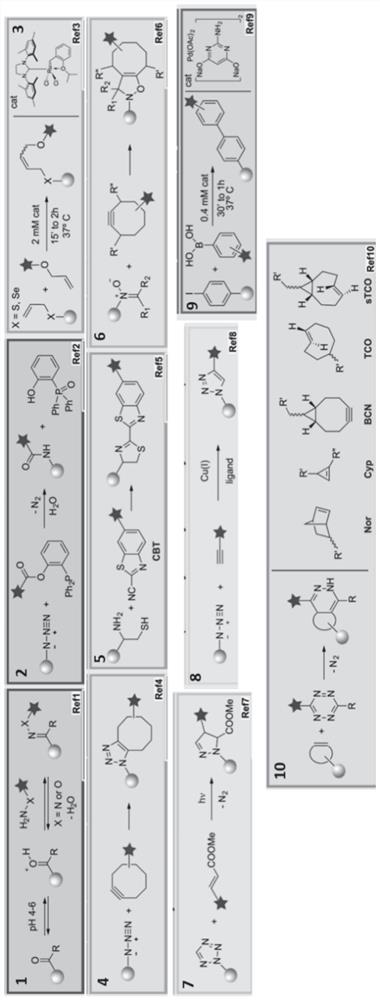
Chemotherapy-sensitizing polypeptide aggregate and its preparation method and application
A technology of aggregates and polypeptides, applied in the field of medicine, can solve the problems of low intracellular effective drug concentration, increase the specificity of cancer cells, etc., and achieve the effects of chemotherapy sensitization and increased permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the design of polypeptide
[0037] 1. Synthesis and covalent coupling of peptides:
[0038] Targeting polypeptide: synthesize a polypeptide capable of targeting and recognizing CAIX, the sequence is YNTNHVPLSPKY;
[0039] Effector peptide: synthesize a peptide with assembly behavior, the sequence is APIAQKDELEKLVFFAEC;
[0040]Experimental instruments and materials: dimethylformamide (DMF), piperidine, resin, dichloromethane (DCM), ninhydrin reaction reagent (ninhydrin, vitamin C, phenol), tetramethyluronium hexafluorophosphate (HBTU), hexahydropyridine, triisopropylsilane TIS, ethanedithiol (EDT), anhydrous ether, trifluoroacetic acid (TFA), N-methylmorpholine (NMM), methanol, various amino acids, Fmoc-e-Acp-OH, FITC, peptide solid-phase synthesis tubes, etc.
[0041] Solution preparation: deprotection solvent - hexahydropyridine: DMF = 1:4; reaction solution - NMM:DMF = 1:24; lysate - TFA (92.5%) TIS (2.5%) EDT (2.5%); Ninhydrin test solution—one dro...
Embodiment 2
[0047] Example 2: The targeting polypeptide 1-FITC can specifically bind to renal cancer cells
[0048] Human kidney cancer tissue samples were obtained from the Tumor Specimen Bank of the Urology Department of the Fourth Affiliated Hospital of Harbin Medical University. The specimens were human renal normal tissue and renal clear cell carcinoma tissue. CAIX is highly expressed in more than 95% of renal clear cell carcinomas, while normal renal tissues hardly express this protein. In order to verify the specificity of the targeting polypeptide 1-FITC, we selected 5 cases (n=5) of each tumor and normal tissue for tissue paraffin-fixed sectioning and co-localization of the targeting polypeptide. The specific operation method:
[0049] 1. Fixation: tissue block, the size of a soybean grain, soaked in 4% paraformaldehyde (cell cryopreservation tube, add more fixative), fixation time > 2 days, rinse with tap water for 2 hours.
[0050] 2. Dehydrated and transparent
[0051] 3. M...
Embodiment 3
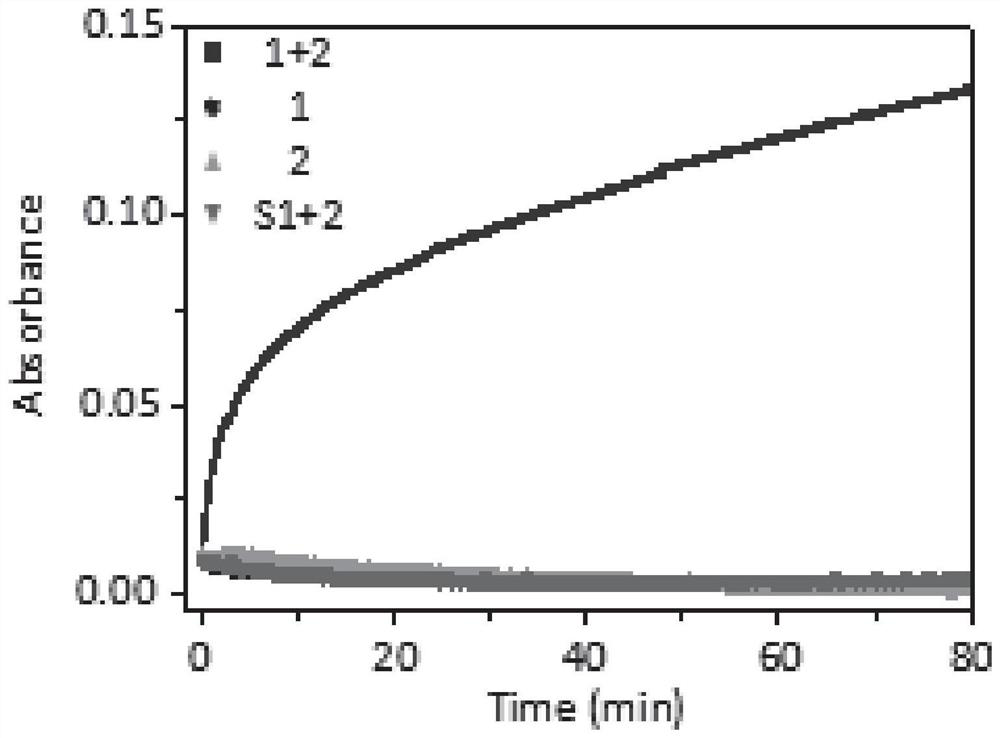
[0068] Example 3: After the polypeptide reacts (1+2), it can remain in the cell level and animal level for a long time
[0069] The cells selected for the experiment were the kidney cancer cell line SK-RC-52 with high expression of CAIX, and the cells were treated with 1-FITC, 2-FITC and 1+2-FITC respectively. Observing the cells under a confocal microscope found that the fluorescence retention time of 1+2-FITC was significantly longer than that of 1-FITC and 2-FITC alone ( Figure 5 ). Subsequently, kidney cancer cells were used to establish mouse xenograft tumors, and 1x10 6 The cells were subcutaneously injected into the right leg of the mouse, and the tumor formed after 2 weeks (n=3). Peptide 1-Cy, polypeptide 2, and polypeptide 1+2 were injected into the rat tail vein respectively, and imaged with IVIS small animal imager. The results showed that the tumor retention ability of polypeptide 1+2 was stronger than that of other groups ( Image 6 ). Then the mice were kill...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com