Composition for treating and/or preventing influenza, method and application thereof
A technology for influenza virus and influenza A, which is applied in the field of protein engineering to achieve the effect of high safety, resistance to resistant strains, and inhibition of virus infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
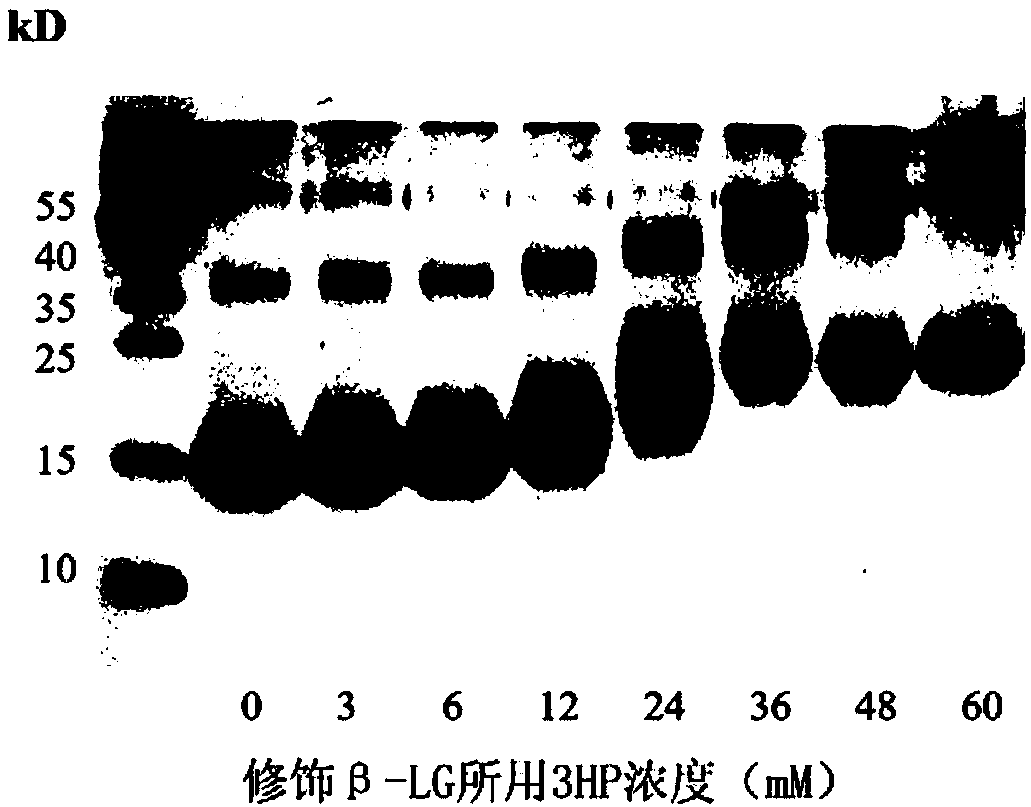
[0063] The invention provides a method for preparing an acid-anhydride-treated protein that has preventive and control effects on various subtypes of seasonal influenza viruses and highly pathogenic influenza viruses. The steps are as follows:
[0064] Dissolve 3-hydroxy-phthalic anhydride powder in dimethyl sulfoxide to make a 1M anhydride solution; use disodium hydrogen phosphate dodecahydrate powder to prepare a 0.1M phosphate solution with a pH of 8.5, and add 400 mg of β-lactoglobulin (β-lactoglobulin, β-LG) powder was dissolved in 20 mL of phosphate solution; 250 μL of 1 M acid anhydride solution was added to the β-LG solution, repeatedly blown and mixed, and the pH was maintained at 9.0, Incubate for 20 minutes, repeat 6 times in total, place at 25°C for 1-2 hours for the last time, so that the protein is completely modified; dialyze the anhydrided protein with pH 7.4 phosphate buffer saline (PBS), change the medium twice during the period, dialyze for 60 Remove the imp...
Embodiment 1
[0072] Example 1. Preparation of bovine β-lactoglobulin treated with acid anhydride
[0073] (1) 3-hydroxy-phthalic anhydride (3-hydroxy phthalic anhydride, HP) powder (purchased from Sigma, product number 320064) was dissolved in dimethyl sulfoxide to prepare a 1M anhydride solution;
[0074] (2) Prepare a 0.1 M phosphate solution with pH 8.5 with disodium hydrogen phosphate dodecahydrate powder, and dissolve 400 mg of bovine β-lactoglobulin (β-lactoglobulin, β-LG) powder (purchased from Sigma, product number L8005) In 20mL phosphate solution;
[0075] (3) Add 250 μL of 1M acid anhydride solution into the β-LG solution (in step (2)), repeatedly blow and mix, adjust the pH to 8.5 with 1M NaOH solution, let stand at 25°C for 20 minutes, repeat 6 times , the last time at 25 ° C for 1-2 hours, so that the protein is completely modified;
[0076] (4) Dialyze the acid-anhydrided protein with phosphate buffered saline (PBS) at pH 7.4, and use a 3.5KD dialysis bag for dialysis, and...
Embodiment 2
[0080] Embodiment 2. detect the concentration of acid anhydride protein with BCA method
[0081] (1) Dilute the anhydrided protein prepared in Example 1 with PBS at a ratio of 10 times, 50 times, and 100 times; and dilute the 2 mg / mL protein standard sample (BSA) with PBS solution to keep the protein 0, 0.1, 0.2, 0.4, 1, 2 mg / mL concentration;
[0082] (2) Add 10 μL of the diluted sample and different concentrations of standard substances into a 96-well plate, and then add 15 μL of PBS to each well to set up 3 duplicate wells;
[0083] (3) Mix liquid A and liquid B in the BCA detection kit (purchased from Takara, product number T9300A) at a ratio of 50:1, add 200 μL in each well to the sample to be tested and the standard, and repeatedly blow and mix, Incubate at 37°C in the dark for 0.5 hours;
[0084] (4) Detect the absorbance of each sample at 562nm with a microplate reader;
[0085] (5) Use GraphPad Prism 5.0 software to draw a scatter diagram of standard substance conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com