Method for synthesizing diene compounds based on aldehyde-ketone condensation reaction
A technology of ketone compounds and aldehyde compounds, applied in the field of diolefin synthesis, which can solve the problems of low yield, easy deactivation of catalysts, high reaction temperature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0138] The preparation method of the supported heteropolyacid compound catalyst is as follows: at room temperature to 60°C and under stirring conditions, the carrier is added to the catalyst mother liquor, impregnated in equal volume, stirred for 6 to 24 hours, and then filtered, and the obtained solid catalyst is in the drying in an oven at 80-120° C. for 5-10 hours to obtain a supported catalyst. The catalyst preparation temperature is preferably room temperature-50°C; the stirring time is preferably 5-18 hours, more preferably 10-15 hours; the drying temperature is preferably 100-120°C; the drying time is preferably 7-10 hours. Among them, the carrier is preferably commercial Al 2 o 3 , SiO 2 , Molecular sieve series, no need to deal with. The molecular sieve series can specifically be one or more of ZSM, BEA, SBA, MCM, SAPO, AlPO, 3 / 4 / 5A molecular sieve, β molecular sieve and other series of molecular sieves, more preferably Al 2 o 3 , SiO 2 , ZSM, SBA, MCM, SAPO or ...
Embodiment 1
[0157] 1. Condensation reaction and catalyst
[0158] Acetone and paraformaldehyde weighed a total of 21g according to 7:3 (molar ratio), placed in a 100mL stainless steel reaction kettle with a PTFE liner, and added 0.3g Mg(OH) 2 20g of methanol / water (mass ratio 5:1) solution, sealed and stirred at 50°C for 10h, extracted with 3×10mL dichloromethane after the reaction, combined organic phases with rotary evaporation to obtain the condensation product, and the conversion rate of paraformaldehyde was ~93, the condensation product yield is ~82%;
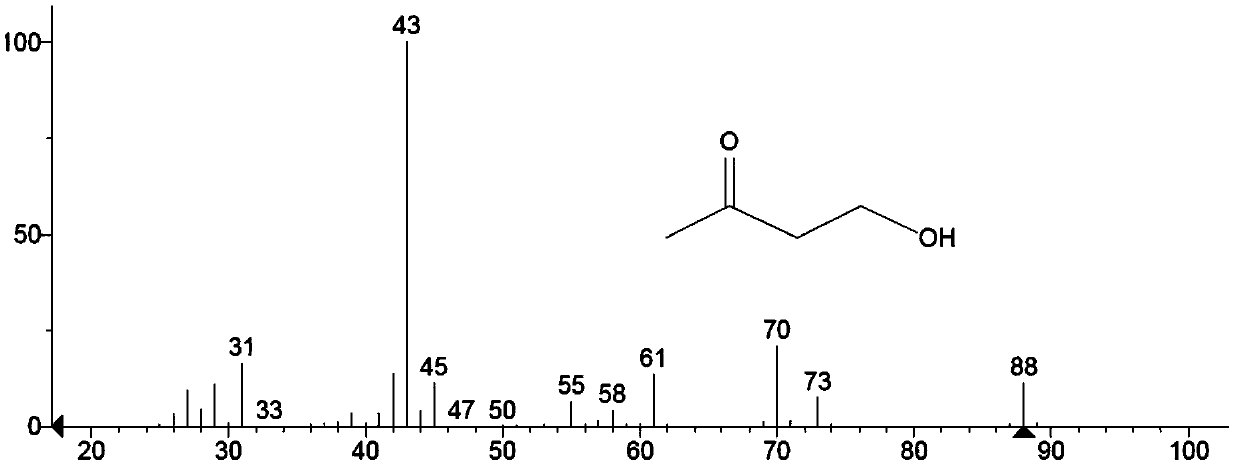
[0159] The condensation product prepared in Example 1 of the present invention was characterized.
[0160] see figure 2 , figure 2 It is the MS spectrum of the condensation product prepared in Example 1 of the present invention.
[0161] 2. Reduction reaction and catalyst
[0162] Take 4g of the condensation product and place it in an ice bath in a 250mL round bottom flask, add a total of 50mL of tetrahydrofuran / water (volume r...
Embodiment 2
[0173] 1. Condensation reaction and catalyst
[0174] Butanone and formaldehyde 7:1 (molar ratio) weighed a total of 24g, placed in a 100mL stainless steel reactor with tetrafluoro liner, added 0.5g NH 4 -ZSM-5 catalyst and 20g methanol / water (mass ratio 5:1) solution, sealed and stirred at 35°C for 4h, extracted with 3×10mL dichloromethane after the reaction, combined organic phase and rotary evaporation to obtain the condensation product, formaldehyde The conversion rate was ~96%, and the condensation product yield was ~85%;
[0175] NH used 4 -The preparation of the ZSM-5 catalyst is as follows: under stirring conditions, 10g ZSM-5 is added to 100mL of prepared 20% ammonia water, stirred at room temperature for 12h, then filtered, and the resulting solid is placed in an oven at 110°C, and the drying time is preferably Be 8h, obtain catalyst;
[0176] 2. Reduction reaction and catalyst
[0177] Take 4g of the condensation product and place it in an ice bath in a 250mL ro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com