Lysosome targeted fluorescent dye based on nitrogen heterocyclic structure as well as preparation method and application of lysosome targeted fluorescent dye
A technology of fluorescent dyes and lysosomes, applied in the preparation of azo dyes, test samples, organic dyes, etc., to achieve the effects of simple synthesis, improved water solubility, and good photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Synthesis of Dye Dansyl-1,4,7,10-tetraazacyclododecane (DS-C)
[0039]
[0040] Dissolve 638.7mg of 1,4,7,10-tetraazacyclododecane (3.71mmol) and 512.3mg of anhydrous potassium carbonate (0.371mmol) in 20mL of anhydrous acetonitrile, stir at room temperature for 30min; dissolve 250mg A solution of dansyl chloride (0.927 mmol) in 10 mL of anhydrous acetonitrile was added dropwise to the above reaction system, and the stirring reaction was continued at room temperature for 3 h. After the reaction was completed, the potassium carbonate powder was removed by suction filtration, and the acetonitrile was removed with a vacuum rotary evaporator. The obtained green sticky residue was purified by column chromatography to obtain the target product DS-C, and the eluent was CH 2 Cl 2 / CH 3 OH (V / V=10:1). 1 H NMR (400MHz, CDCl 3 ):δ8.56(d,1H), 8.38(d,1H),7.98(d,1H),7.52(m,2H),7.19(d,1H),3.62(t,4H),3.20(t, 4H),3.04(t,4H), 2.88-2.91(m,10H). 13 C NMR (100MHz, CDCl ...
Embodiment 2
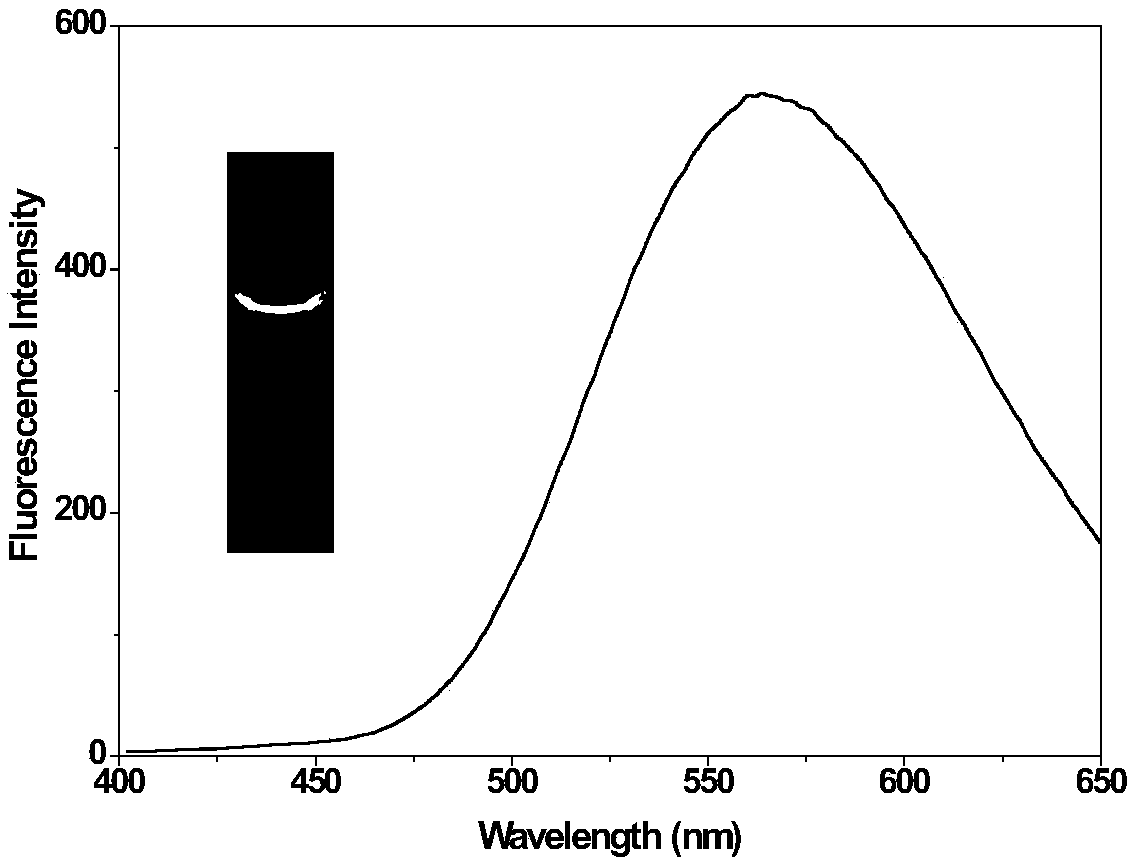
[0041] Embodiment 2: the fluorescence spectrum of dyestuff DS-C in PBS buffer solution
[0042] Dissolve DS-C in ethanol to prepare a stock solution with a concentration of 10mmol / L. 2 μL DS-C was taken from the stock solution and added to 2 mL of PBS buffer solution (pH=7.4, 10 mM), so that the final concentration of the probe was 10 μM, and the fluorescence emission of the dye DS-C was measured at an excitation wavelength of 405 nm. And under the irradiation of 365nm ultraviolet lamp, take the fluorescent picture of dyestuff with digital camera. Fluorescence spectra and fluorescence photos of dyes such as figure 1 shown.
Embodiment 3
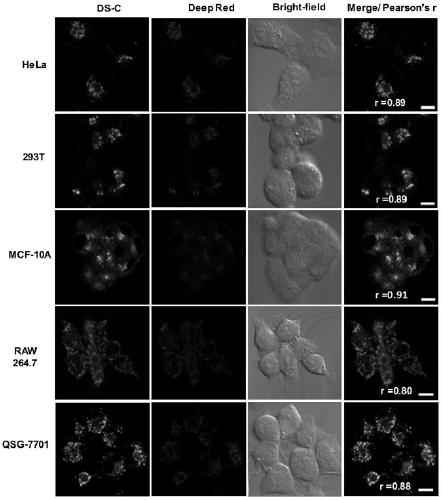
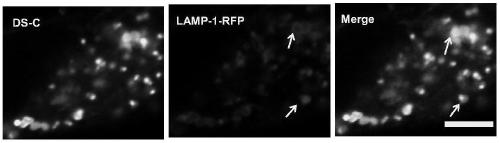
[0043] Example 3: Co-localization of the dye DS-C with the commercial lysosomal dye LysoTracker Deep Red in different cells
[0044] The lysosomal dye Deep Red was added to different types of cells respectively, at 37°C, 5% CO 2 After culturing for 30 min under the condition, wash once with medium or (PBS) to remove residual Deep Red. Subsequently, the fluorescent dye DS-C prepared in Example 1 was added to the above-mentioned cells to make the final concentration 5 μM. After culturing for 10 minutes, laser confocal imaging was performed. The imaging results are shown in figure 2 . It can be seen from the figure that the dye DS-C and Deep Red can overlap perfectly, indicating that the dye has the ability to specifically target lysosomes and is universal. The reason why the dye can specifically target the lysosome is that the nitrogen heterocyclic substituent is weakly basic, and the environment in the cell lysosome is weakly acidic, and the dye has the tendency to accumulat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com