A kind of preparation method of 17a-prednisolone dehydroxyacetate product
A technology of prednisolone dehydroxyacetate and prednisolone acetate, which is applied in the production process field of synthesizing 17a-prednisolone dehydroxyacetate, can solve the problems of high production cost, low synthesis yield and many side reactions and other problems, to achieve the effect of simple and convenient production operation, high yield and reduced preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
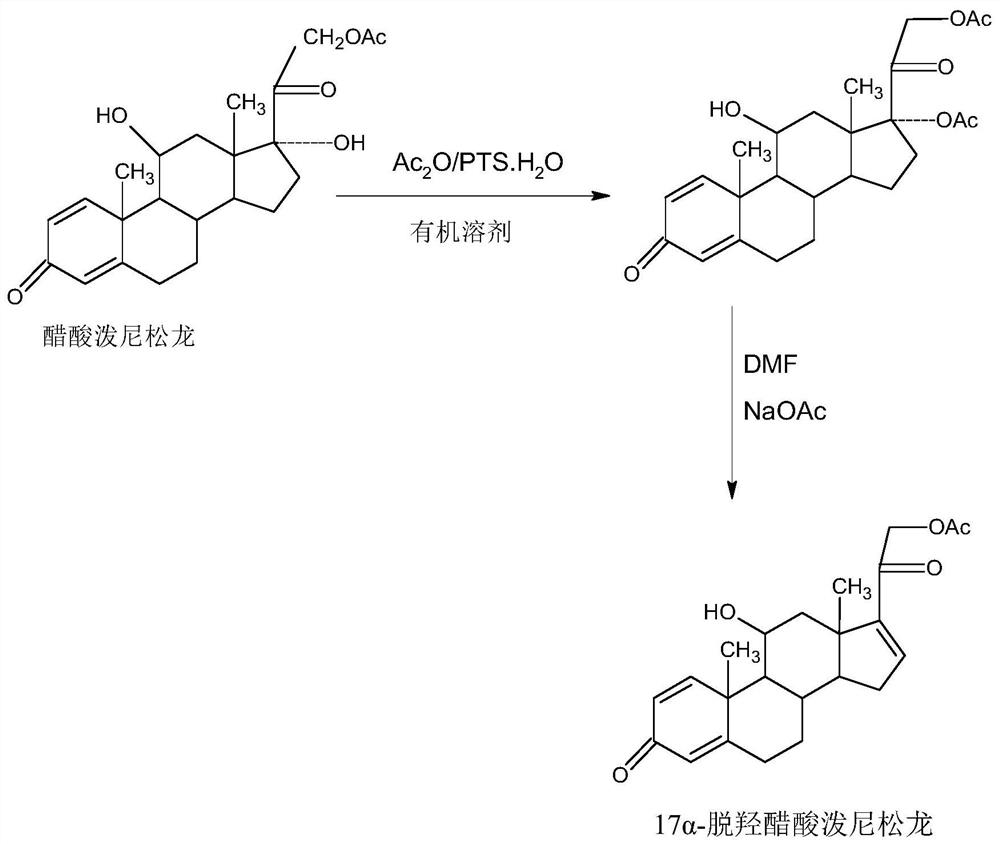
[0018] Further, the specific operation steps of the preparation method of 17a-prednisolone dehydroxyacetate are as follows:
[0019] Step A, preparation of protective material: dissolve prednisolone acetate in an organic solvent, add alkali catalyst and trimethylchlorosilane, stir and react at 10-50°C for 3-4 hours, after the reaction, evaporate under reduced pressure Organic solvent, then adding tap water for water analysis, filtering, washing, and drying to obtain the protected product: 11-trimethylsilyl etherified prednisolone acetate, with a weight yield of about 120-125%;
[0020] Step B. Preparation of 17a-prednisolone dehydroxyacetate: Dissolve the protective compound prepared in the above A into an organic solvent, add an organic base catalyst, and keep warm at 10-100°C and feed SO 3 Gas dehydration reaction for 6-12 hours, TLC to confirm the reaction end point, after the reaction, directly add acid aqueous solution to hydrolyze and deprotect at 10-100 ° C, TLC to conf...
Embodiment 1
[0029] Step A, preparation of protection:
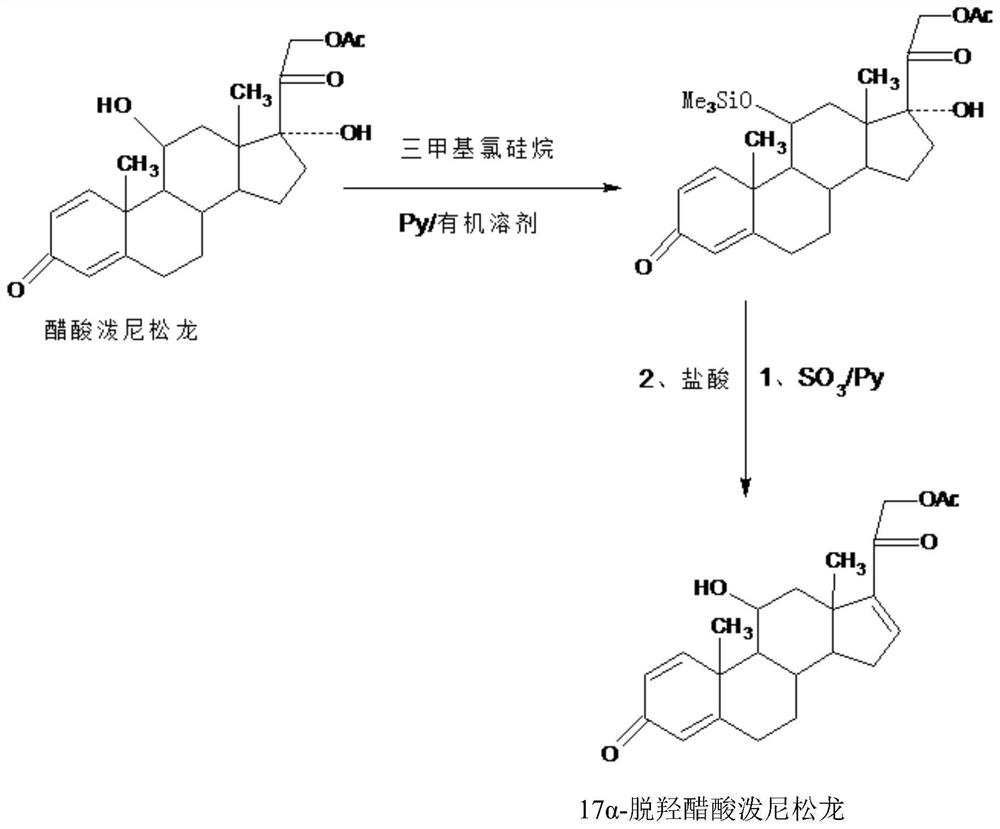
[0030] In a 1000ml three-necked bottle, add 100g prednisolone acetate, 600ml dichloromethane, 100g pyridine, stir to dissolve the solid completely, slowly add 80g trimethylchlorosilane dropwise at 20-25℃, about 1.5-2 After adding in 1 hour, continue to insulate and stir the reaction at 20-25°C for 3 to 4 hours after dropping, and confirm the end point of the reaction by TLC. After the reaction, 90-95% of the organic solvent dichloromethane is recovered by vacuum distillation below 30°C, and then Add 600ml of tap water, stir and crystallize at 10-15°C for 3-4 hours, filter, send the filtrate to a wastewater treatment tank for treatment, wash the filter cake with water, and dry it below 60°C to obtain the protected product: 11-trimethylsilyl etherified acetic acid Prednisolone 123.6g, HPLC content 97.6%, weight yield 123.6%;
[0031] B, preparation 17a-prednisolone dehydroxyacetate:
[0032] In a 2000ml three-necked flask, add 100g o...
Embodiment 2
[0036] A. Preparation of protective materials:
[0037] In a 1000ml three-necked bottle, add 100g of prednisolone acetate, 600ml of chloroform, and 80g of triethylamine, stir to dissolve the solid completely, and slowly add 80g of trimethylchlorosilane dropwise at 20-25°C, about 1.5 - After adding in 2 hours, continue to insulate and stir the reaction at 20-25°C for 3-4 hours, and confirm the end point of the reaction by TLC. After the reaction, recover 90-95% of the organic solvent chloroform by distillation under reduced pressure below 30°C , then add 600ml of tap water, stir and crystallize at 10-15°C for 3-4 hours, filter, send the filtrate to the wastewater treatment tank for treatment, wash the filter cake with water, and dry below 60°C to obtain the protected product: 11-trimethylsilyl ether Prednisolone acetate 123.2g, HPLC content 97.2%, weight yield 123.2%;
[0038] B, preparation 17a-prednisolone dehydroxyacetate:
[0039] In a 2000ml three-neck flask, add 100g of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com