Preparation method of micamba methyl ester
A technology of dicamba methyl and methanol, which is applied in the field of pesticides, can solve the problems of complex synthesis process, low purity of dicamba methyl, and high equipment requirements, and achieve the effects of high reaction selectivity, fast reaction rate and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
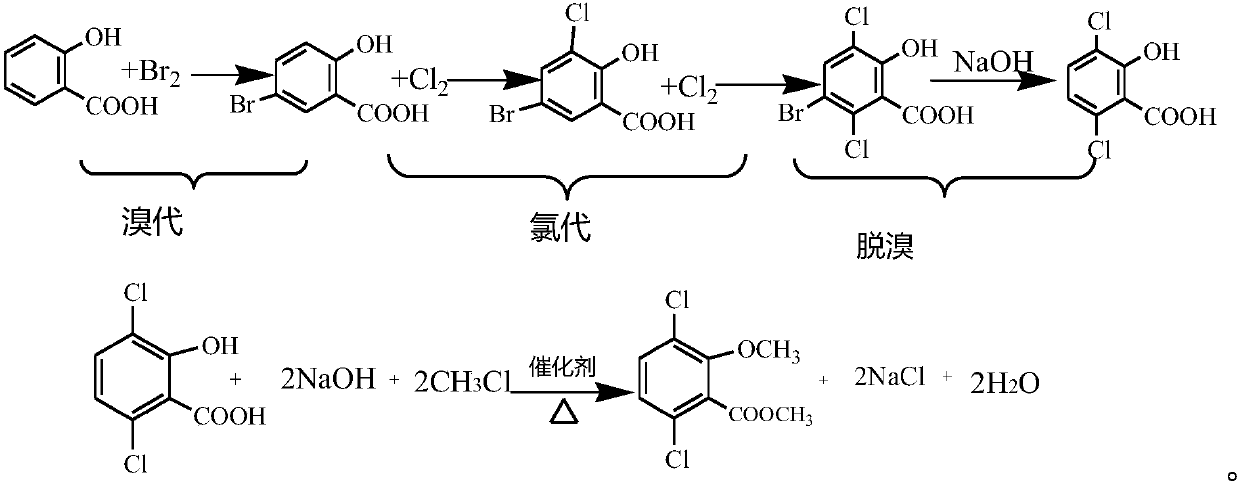
[0078] Put 138g of salicylic acid into 1000g of 95% sulfuric acid at 5°C, slowly add 80g of bromine dropwise, raise the temperature to 20°C for 2 hours after the dropwise addition, then raise the temperature to 45°C, and start to introduce chlorine gas, all of which will generate 5-bromo- For 3-chlorosalicylic acid, stop the chlorine flow, slowly add 400g of sulfur trioxide, add 0.15g of iodine, maintain the temperature at 35°C, start to pass in chlorine gas, all of which will generate 5-bromo-3,6-dichlorosalicylic acid in the central control When it is acidic, stop feeding chlorine gas. The reaction solution was poured into ice water, the sulfuric acid concentration of the system was diluted to 30%, filtered, washed with water and dried to obtain 237.8g of 5-bromo-3,6-dichlorosalicylic acid with a yield of 75.0% and a purity of 90.2% (based on salicylic acid acid meter).
[0079] For debromination reaction, add 5-bromo-3,6-dichlorosalicylic acid into 1075g of 10% liquid caus...
Embodiment 2
[0082] Put 138g of salicylic acid into 1000g of 95% sulfuric acid at 5°C, slowly pass in 80g of hydrogen bromide, raise the temperature to 20°C for 2 hours after the dropwise addition, then raise the temperature to 45°C, start to pass in chlorine gas, all of which will generate 5-bromo When -3-chlorosalicylic acid, stop chlorine, slowly add 400g of sulfur trioxide, add 0.15g of iodine, maintain the temperature at 35°C, start to pass in chlorine gas, all 5-bromo-3,6-dichloro water will be generated in the central control When salicylic acid is used, stop feeding chlorine gas. The reaction solution was poured into ice water, the sulfuric acid concentration in the system was diluted to 30%, filtered, washed with water and dried to obtain 238.1 g of 5-bromo-3,6-dichlorosalicylic acid. Yield 75.3%, purity 90.1%.
[0083] For debromination reaction, add 5-bromo-3,6-dichlorosalicylic acid into 1075g of 10% ammonia water, raise the temperature to 40°C until it is fully dissolved, aft...
Embodiment 3
[0086] Put 138g of salicylic acid into 1000g of 95% sulfuric acid at 5°C, slowly pass in 80g of hydrogen bromide, raise the temperature to 20°C for 2 hours after the dropwise addition, then raise the temperature to 45°C, start to pass in chlorine gas, all of which will generate 5-bromo When -3-chlorosalicylic acid, stop chlorine, slowly add 400g of sulfur trioxide, add 0.15g of iodine, maintain the temperature at 35°C, start to pass in chlorine gas, all 5-bromo-3,6-dichloro water will be generated in the central control When salicylic acid is used, stop feeding chlorine gas. The reaction solution was poured into ice water, the sulfuric acid concentration in the system was diluted to 30%, filtered, washed with water and dried to obtain 238.1 g of 5-bromo-3,6-dichlorosalicylic acid. Yield 75.2%, purity 90.2%.
[0087] For debromination reaction, add 5-bromo-3,6-dichlorosalicylic acid into 1075g of 10% potassium hydroxide, raise the temperature to 30°C until it is fully dissolve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com