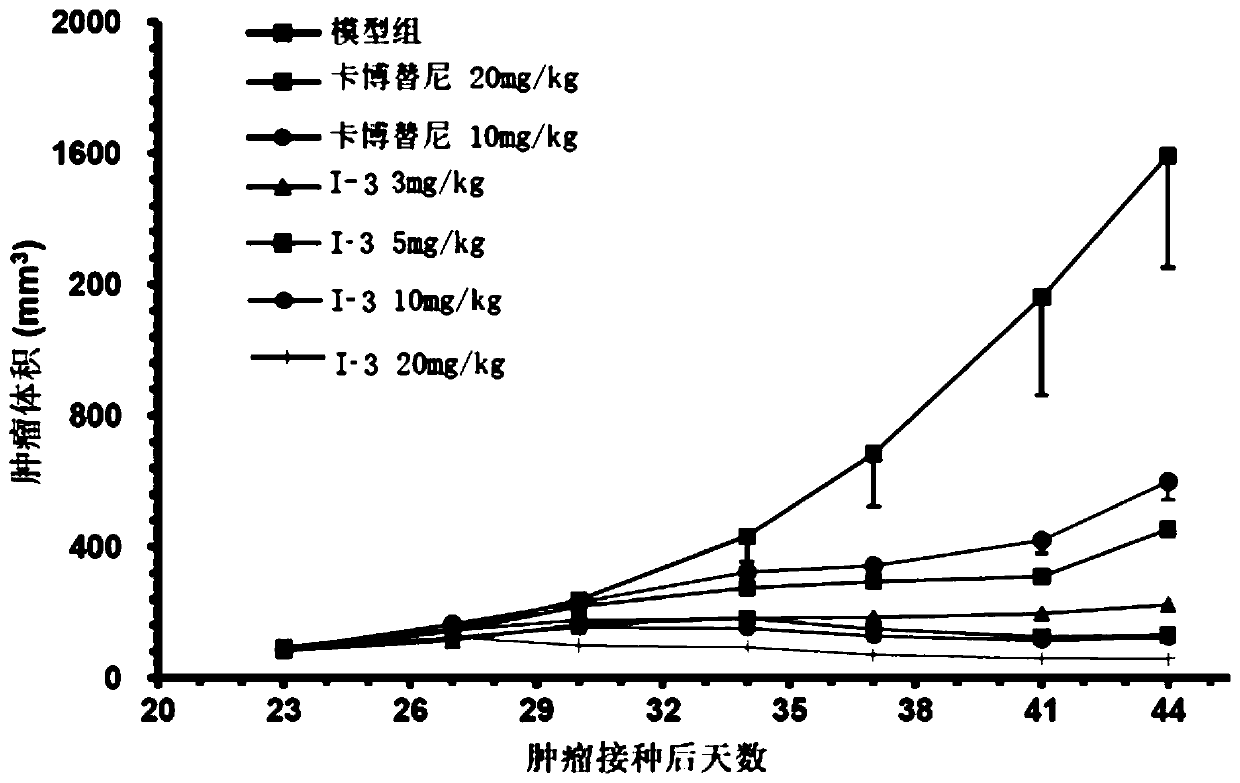
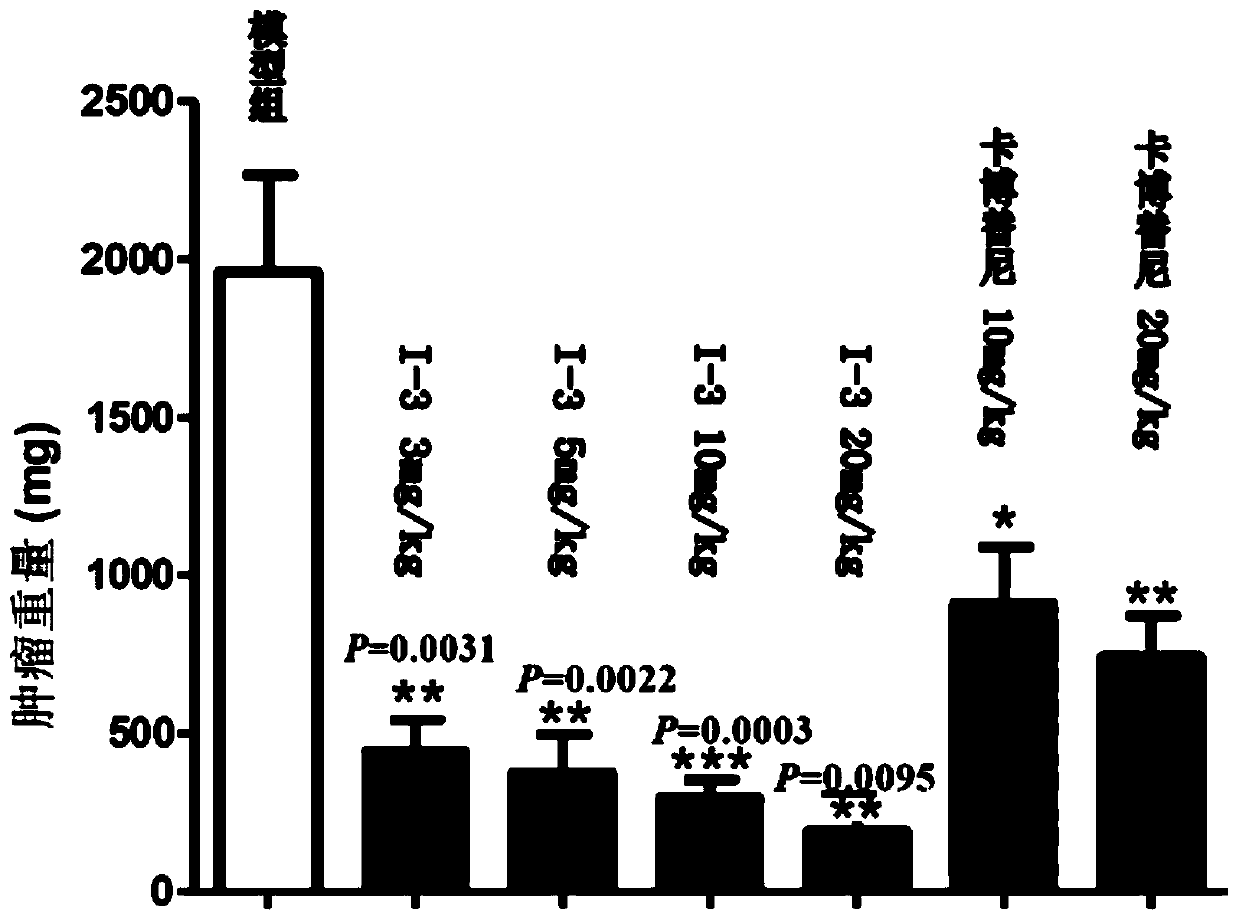
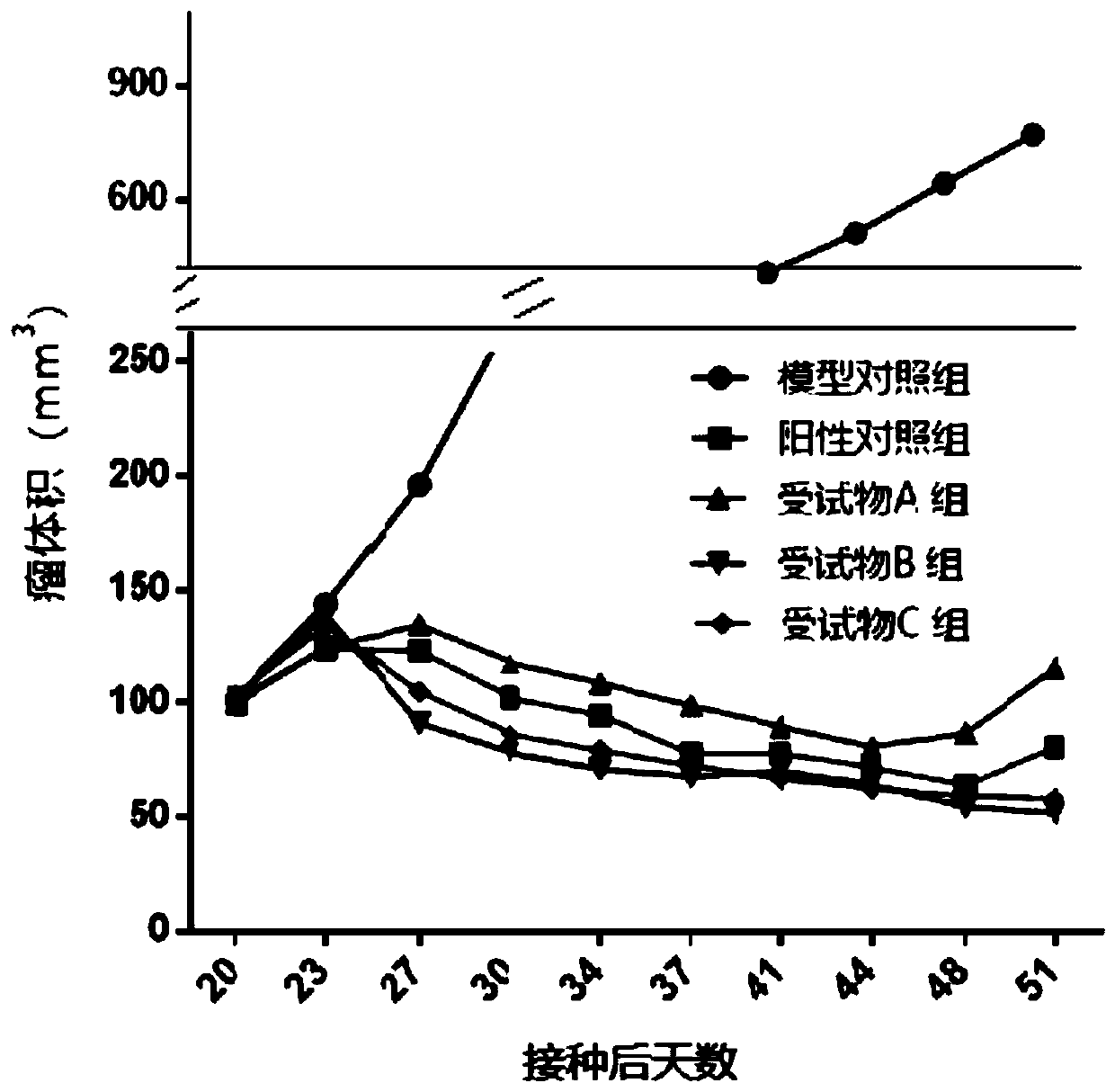
Quinoline derivative, pharmaceutically acceptable salt or solvate thereof, application thereof, medicines and pharmaceutical compositions
A technology of solvates and derivatives, which is applied in the direction of drug combinations, active ingredients of heterocyclic compounds, and medical preparations containing active ingredients, etc., can solve the problems that the anti-tumor effect needs to be improved, so as to prolong the time of drug resistance and improve Curative effect, excellent anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The quinoline derivatives described in the embodiments of the present invention can be prepared by various methods, and the embodiments of the present invention provide a method for preparing the quinoline derivatives with a relatively high yield, comprising the following steps:
[0053] (1) compound (Ic) is obtained by reacting compounds (Ia) and (Ib);
[0054]
[0055] (2) obtain compound (Ie) by reaction of compound (Ic) and (Id);
[0056]
[0057] (3) obtain described quinoline derivative by reaction of compound (Ie) and compound (If);
[0058]
[0059] R 1 selected from methyl, deuterated methyl, any structure in
[0060] R 2 Any one selected from F, Cl, Br, I, H;
[0061] R 3 selected from F, CHF 2 、CF 3 , any one of Cl.
[0062] Compound (Ia) and Compound (Ib) are pharmaceutical intermediates and can be purchased. In step (1), compound (Ia) and compound (Ib) are first amidated and then hydrolyzed under basic catalytic conditions to obtain comp...
Embodiment 1
[0084] Compound I-1N-(4-((6-carbamoyl-7-methoxyquinolin-4-yl)oxy)phenyl)-N-(4-fluorophenyl)cyclopropane-1,1 -The synthetic route of dicarboxamide is as follows:
[0085]
[0086] resolve resolution:
[0087](1) Add p-aminophenol (10.9g, 0.1mol) and triethylamine (30.3g, 0.3mol) into dichloromethane (300ml), stir at room temperature to make it into a uniform suspension state (solution A). Methyl 1-(chlorocarbonyl)cyclopropanecarboxylate (17.82 g, 0.11 mol) was dissolved in 100 ml of dichloromethane to form a solution (Solution B). Add solution B dropwise to solution A, stir the reaction at room temperature after the dropwise addition is completed, track the reaction by TLC (thin layer chromatography analysis), and analyze and confirm that the reaction is completed. After the reaction is completed, the solution after the reaction is filtered to remove insoluble impurities, and the organic phase is washed with dilute hydrochloric acid. Wash once, then wash once with brine, d...
Embodiment 2
[0092] Compound I-2N-(4-((6-carbamoyl-7-methoxyquinolin-4-yl)oxy)-2-chlorophenyl)-N-(4-fluorophenyl)cyclopropane- The synthetic route of 1,1-dicarboxamide is as follows:
[0093]
[0094] Synthetic method can refer to embodiment 1, and in step (1) and step (2), equivalent ratio and reaction condition are all constant, and step (3) compound (I-2e) (8.36g, 0.024mol) and 4-chloro- 6-carbamoyl-7-methoxyquinoline (4.72g, 0.02mol) was added to 70ml of DMF, then sodium hydroxide (1.12g, 0.028mol) was added, under nitrogen protection, the reaction was heated and stirred at 60°C, passed TLC followed the reaction. After the analysis confirmed that the reaction was complete, 80 mL of purified water was added dropwise to the reacted solution. After the solid was precipitated, it was filtered with suction, and the filter cake was washed with ethanol to obtain the crude product. The crude product was purified by column chromatography to obtain compound (I-2). 4.28g, yield 39%.
[0095]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com