Synthesis of helicobacter-pylorus O2 serotype O antigen oligosaccharide compound
A technology of Helicobacter pylori and compounds, applied in the direction of carrier binding antigen/hapten components, sugar derivatives, sugar derivatives, etc., can solve problems such as research interference, few products, and insufficient specificity of lipopolysaccharide structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
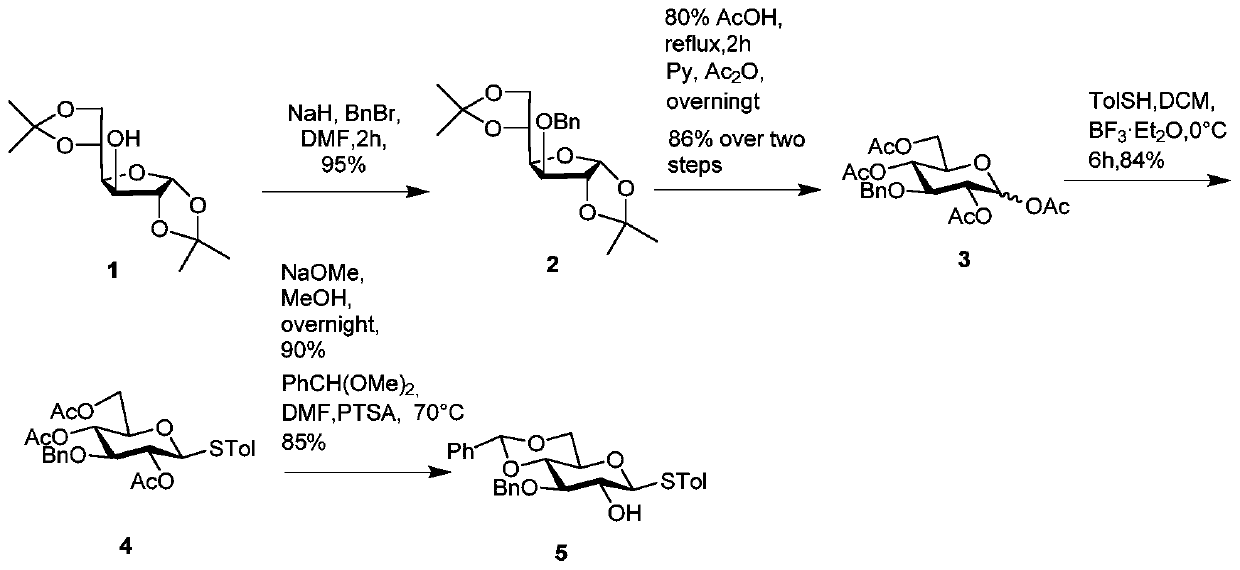
[0088] Synthesis of Sugar Block 5 as figure 1 :
[0089] Such as figure 1 As shown, the commercially available diacetone glucose 1 was used as the starting material to react with benzyl bromide under the action of sodium cyanide to generate 3-OBn glucose compound 2. After removing the propylidene group in 80% acetic acid solution, under the action of pyridine and acetic anhydride, the four newly formed hydroxyl groups were protected with acetyl groups to obtain compound 3. Then compound 3 was reacted in boron trifluoride ether (BF 3 ·Et 2 O) under the effect of reacting with thiophenol to generate 1-glucoside compound 4. Remove the three acetyl groups of compound 4 with methanolic sodium methoxide solution, then use benzaldehyde dimethyl acetal and p-toluene cyclic acid to protect the 4 and 6 hydroxyl groups, leaving the 2 Exposure of hydroxyl groups yields sugar building block 5.
[0090] Specific test operations and steps.
[0091] Compound 2: Dissolve diacetone gluco...
Embodiment 2
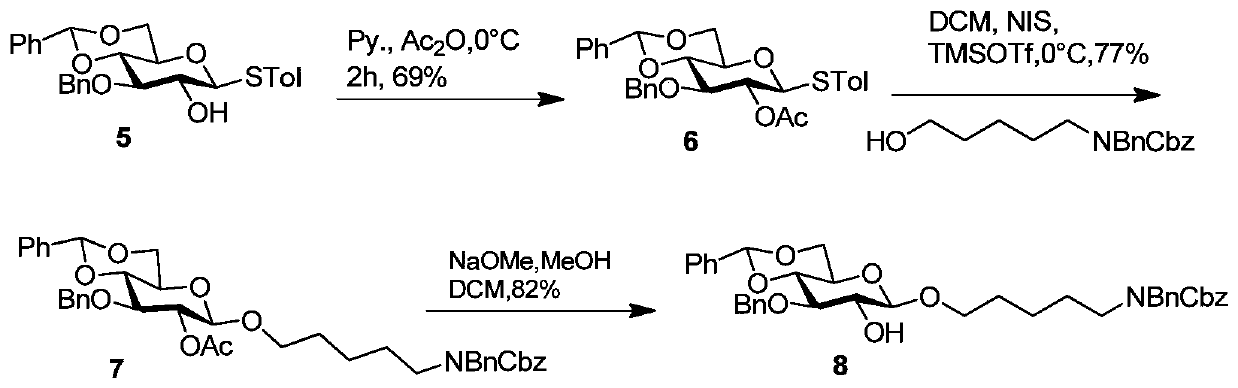
[0098] Synthesis of Sugar Block 8
[0099] The synthesis of sugar block 8 as figure 2
[0100] Such as figure 2 As shown, compound 8 is an analog of compound 5 with an amino linker assembled at the reducing end. Starting from compound 5, first protect 2-OH with acetyl to obtain compound 6, and then use p-methylphenylthio as a leaving group to react with the five-carbon amino linking arm under the accelerator NIS and TMSOTf to obtain compound 7. Finally, 2-OAc of compound 7 was removed with methanol and sodium methoxide to obtain sugar block 8.
[0101] Specific test operation and steps:
[0102] Compound 6: Compound 5 (9.7g, 21mmol) was dissolved in dry pyridine (60ml), and acetic anhydride (40ml) was slowly added in ice bath. The reaction solution was stirred at room temperature for 6 h. After the reaction of the raw materials was detected by TLC, the reaction solution was diluted with dichloromethane, washed with 1M hydrochloric acid solution, extracted with saturated...
Embodiment 3
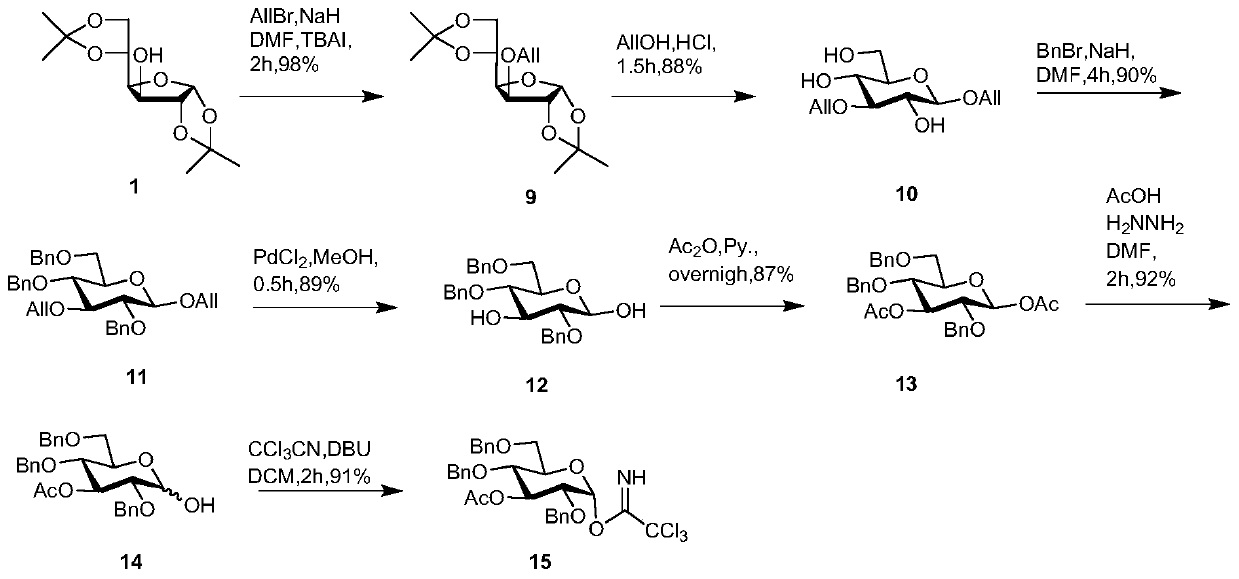
[0106] Synthesis of Sugar Block 15
[0107] Such as image 3 As shown, the diacetone glucose 1 is used as the starting material, and the 3-allyl compound 9 is obtained by reacting with allyl bromide under the action of NaH. Compound 9 undergoes a ring-opening reaction in allyl alcohol added with hydrochloric acid to obtain 1,3-diallyl compound 10. Next, the remaining three exposed hydroxyl groups of glucose were protected with benzyl groups to obtain compound 11. Compound 11 uses palladium chloride in dry methanol to remove allyl groups at positions 1 and 3 exclusively to obtain 1-OH compound 12. The two hydroxyl groups formed in the previous step were protected with acetyl to obtain compound 13. Selective removal of the 1-OAc group under the action of hydrazine acetate gave 1-OH compound 14. Finally, compound 14 was reacted with trichloroacetonitrile and DBU to obtain trichloroacetimide ester glycosyl building block 15.
[0108] Specific test operation and steps:
[010...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com