Method for preparing sodium iron phosphate electrode by using industrial byproduct ferrous sulfate
A technology of ferrous sulfate and sodium ferric phosphate, which is applied in battery electrodes, chemical instruments and methods, circuits, etc., can solve the problem that the thermodynamically stable phase does not have the activity of sodium ion insertion and extraction, and there is no ideal post-treatment recycling route. Sodium iron phosphate cathode material and other problems, to achieve excellent capacity and cycle stability, alleviate disposal problems, and achieve the effect of high electrochemical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
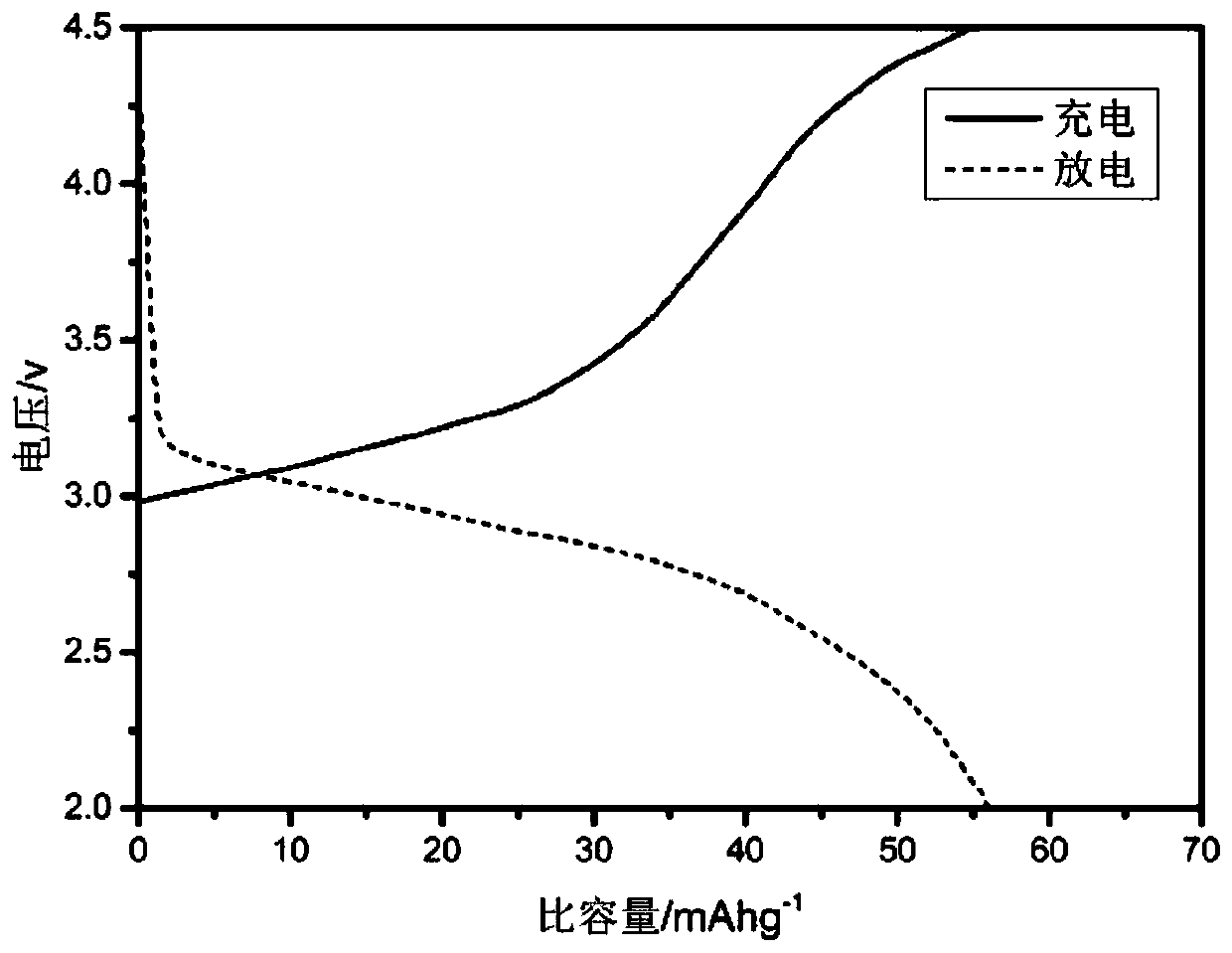
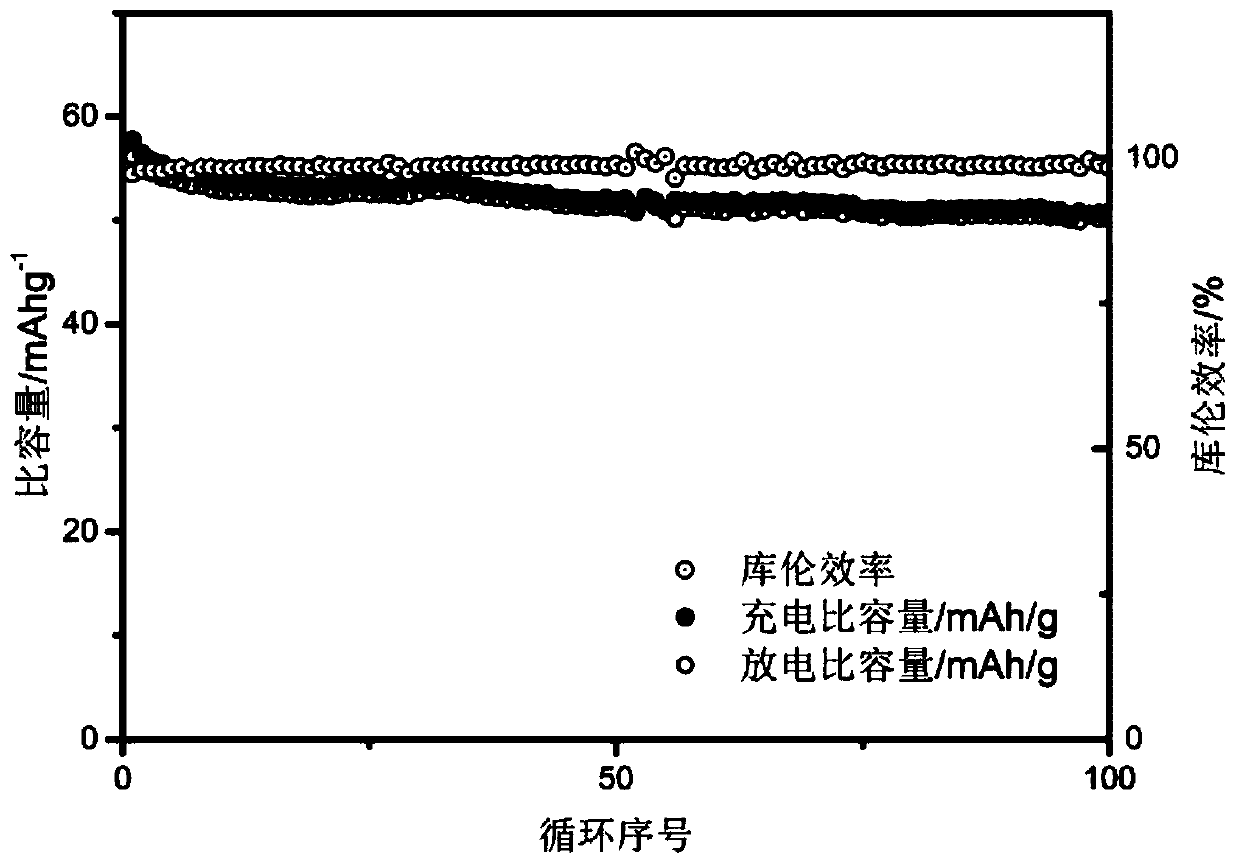
[0037] 3g ferrous sulfate (FeSO 4 ·7H 2 O) dissolved in 50mL phosphoric acid (H 3 PO 4 ), then add 15mL hydrogen peroxide (H 2 o 2 ), then add ammonia water drop by drop while pH monitoring, control the target pH value to 1.5, white precipitate is produced in the solution, collect the precipitate by suction filtration and then use 100mL deionized water and 100mL absolute ethanol to repeatedly suction filter the precipitate three times Finally, the precipitate was dried in an oven at 60°C for 12 hours, and then the dried sample was placed in a tube furnace, and heat-treated at 600°C for 12 hours under a nitrogen atmosphere to remove crystal water and obtain a sample of iron phosphate powder.
[0038] Prepare ferric phosphate powder, PVDF, and conductive carbon black into a mixed powder according to the mass ratio of 8:1:1, add NMP liquid of the same quality as PVDF, and continue stirring for 3 hours to obtain iron phosphate slurry. Use a film coating machine to press 0.3mm ...
Embodiment 2
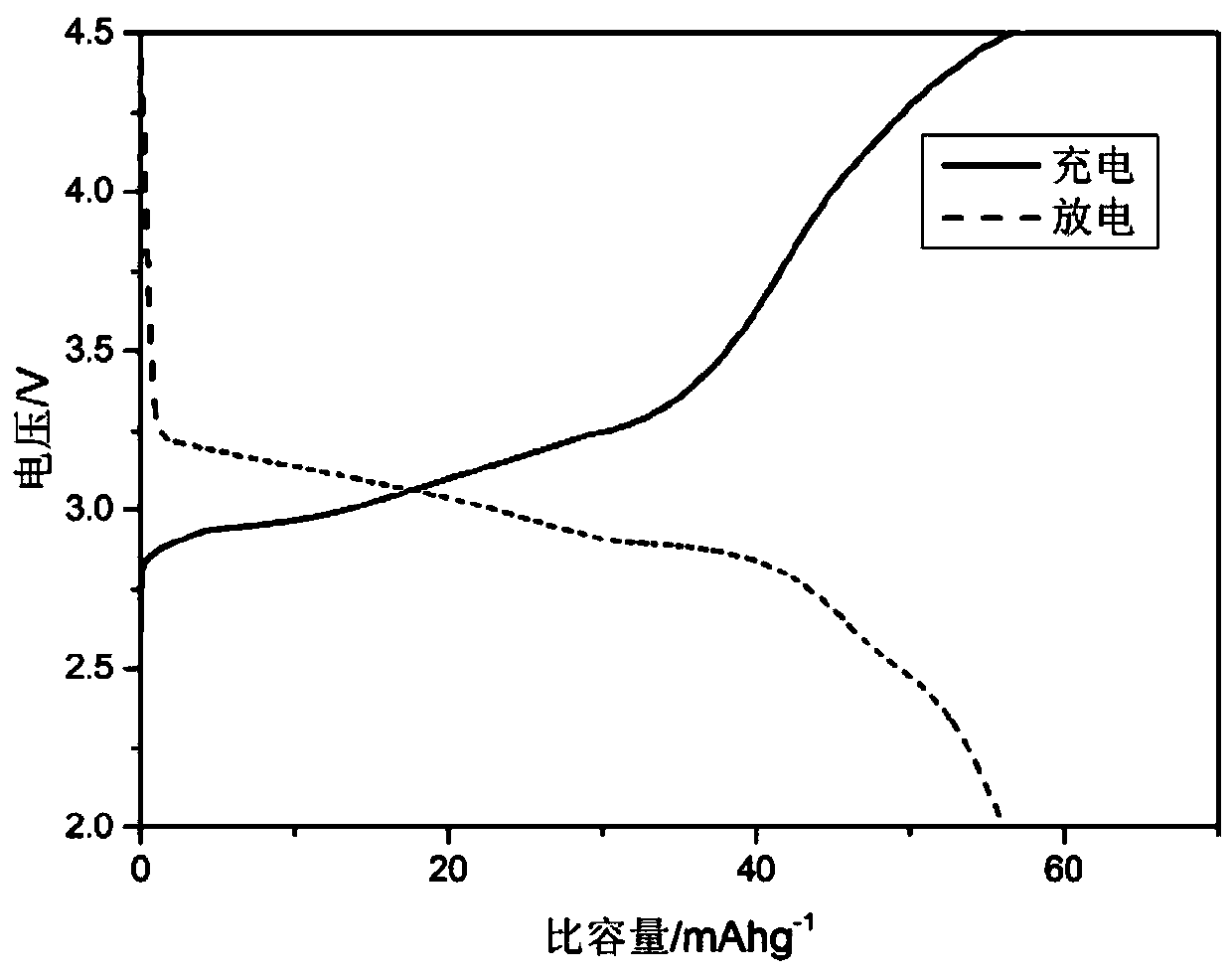
[0042] 3g ferrous sulfate (FeSO 4 ·7H 2 O) dissolved in 50mL phosphoric acid (H 3 PO 4 ), then add 30mL hydrogen peroxide (H 2 o 2 ), then add saturated sodium hydroxide solution drop by drop while pH monitoring, control the target pH value to 1.5, produce white precipitate in the solution, use 100mL deionized water and 100mL absolute ethanol to repeatedly remove the precipitate after collecting the precipitate by suction filtration After three times of suction filtration, the precipitate was dried in an oven at 60°C for 12 hours, and then the dried sample was placed in a tube furnace, and heat-treated at 700°C for 12 hours under a nitrogen atmosphere to remove crystal water and obtain iron phosphate powder sample.
[0043] Prepare ferric phosphate powder, PVDF, and conductive carbon black into a mixed powder according to the mass ratio of 7:2:1, add NMP liquid of the same quality as PVDF, and continue stirring for 3 hours to obtain iron phosphate slurry. Use a film coati...
Embodiment 3
[0047] 3g ferrous sulfate (FeSO 4 ·7H 2 O) dissolved in 50mL phosphoric acid (H 3 PO 4 ), then add 10mL hydrogen peroxide (H 2 o 2 ), then add concentrated ammonia water drop by drop while pH monitoring, and control the target pH value to 1.5, a white precipitate is produced in the solution, collect the precipitate by suction filtration, and then use 100mL deionized water and 100mL absolute ethanol to repeatedly suction-filter the precipitate After three times, the precipitate was dried in an oven at 60°C for 12 hours, and then the dried sample was placed in a tube furnace and heat-treated at 700°C for 12 hours under a nitrogen atmosphere to remove crystal water and obtain a sample of iron phosphate powder.
[0048] Mix iron phosphate powder, PTFE, and acetylene black in a mass ratio of 8:1:1 to make a mixed powder, add isopropanol equal in quality to PTFE, and continue stirring for 3 hours to obtain iron phosphate slurry. Use a coating machine to press 0.3mm The thicknes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com