Spirofluorenexanthene type electron transport material and preparation method and application thereof
An electron transport material, the technology of spirofluorene xanthene, which is applied in the direction of circuits, electrical components, electric solid devices, etc., can solve the problems of poor transmission performance, and achieve the effects of low cost, easy solution processing, and high rigidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
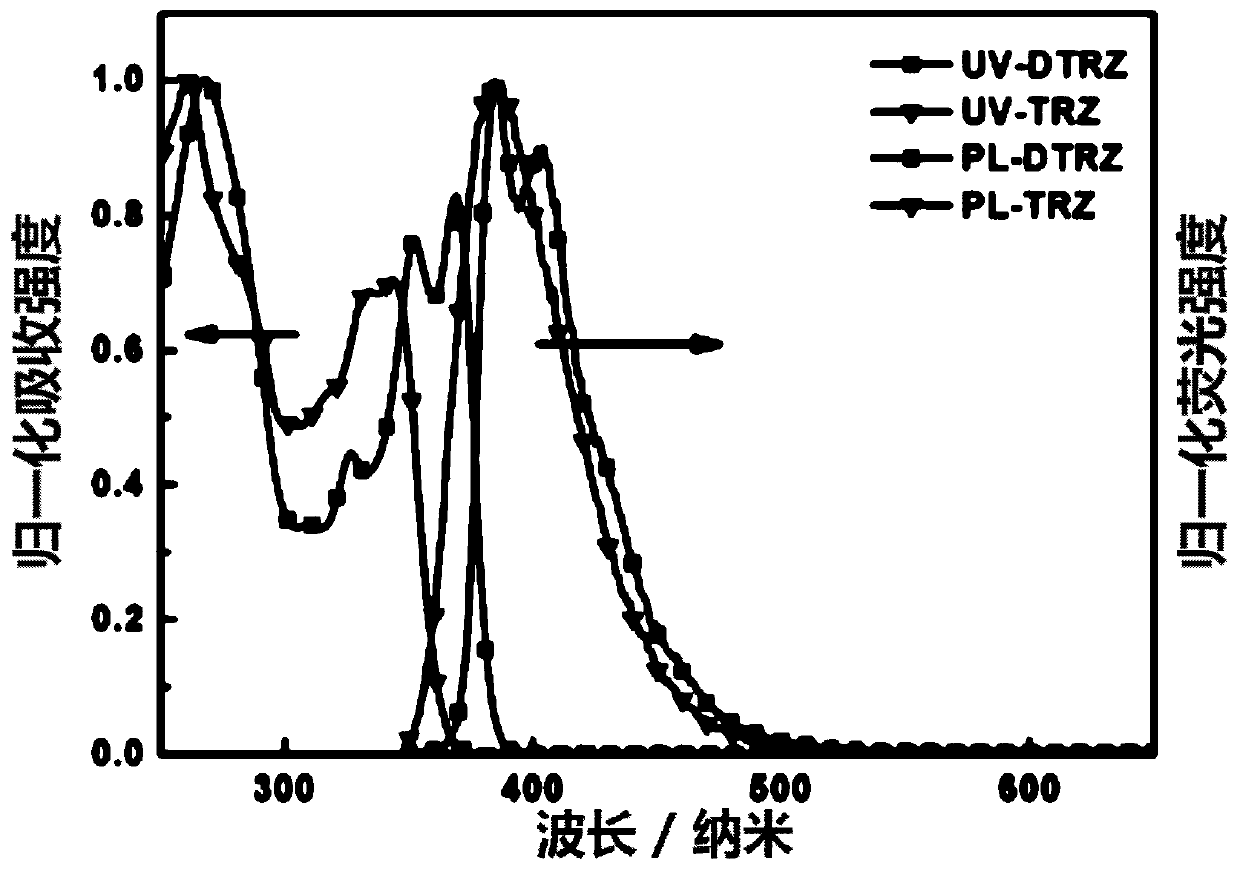
Image
Examples
preparation example Construction
[0059] The present invention also discloses a preparation method of a spirofluorene xanthene-type electron transport material, which specifically includes the following steps:
[0060] S1: The 2-bromospirofluorene xanthene derivative in the general formula I (1) is obtained by palladium-catalyzed Suzuki coupling reaction to obtain 2-(1,3,5-s-triazine)-spirofluorene xanthene derivative thing;
[0061] S2: The 2,7-dibromospirofluorene xanthene derivative in the general formula I (1) is obtained by palladium-catalyzed Suzuki coupling reaction to obtain 2,7-bis(1,3,5-s-triazine)- Spirofluorene xanthene derivatives;
[0062] Its reaction scheme general formula I is:
[0063]
[0064] Among them: L is one of the following structures
[0065]
[0066] Among them, R in the structural formula 3 and R 4 Independently of hydrogen, C1-C12 alkyl, C1-C12 alkoxy, C6-C60 aryl or C3-C60 heteroaryl;
[0067] Ar 1 and Ar 2 Independent of one of the following structural formulas:
...
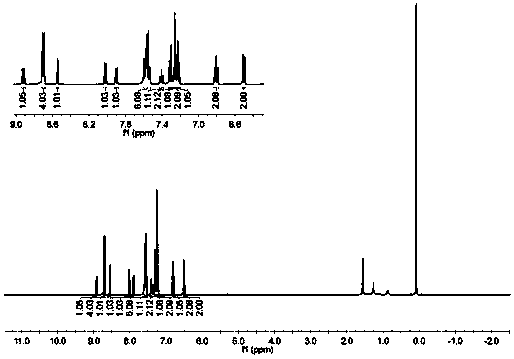
Embodiment 1
[0078] Following formula is the chemical reaction formula of embodiment 1:
[0079]
[0080] Prepare B from A:
[0081] Assemble the two-neck flask, magnet, and condenser tube, leaving only the sample injection port in the sealed system, while bubbling the refined 1,4-dioxane to remove the air inside. Raw material compound A (1.00g, 2.44mmol), pinacol diboronate (0.92g, 3.62mmol), potassium acetate (0.72g, 7.32mmol), 1,1'-bisdiphenylphosphinoferrocene Palladium chloride (0.36g, 0.48mmol) was added into the flask, the system was sealed, and the reaction flask was wrapped with tinfoil. Vacuum blow nitrogen 2-3 times, inject 5 mL of 1,4-dioxane into the reaction flask. 100 ℃ oil bath stirring and heating reaction for 24h, after the reaction was completed, the organic layer was extracted with dichloromethane, the organic phase was combined, dried with anhydrous sodium sulfate, concentrated by rotary evaporation to remove the solvent, separated and purified by column chromatog...
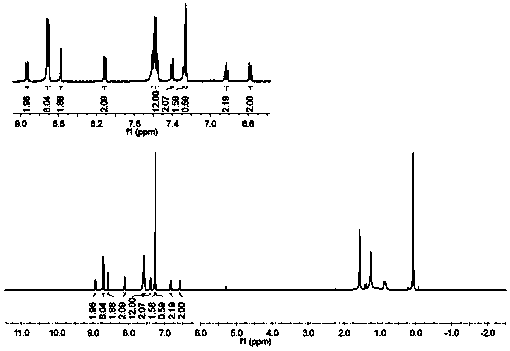
Embodiment 2
[0085] Following formula is the chemical reaction formula of embodiment 2:
[0086]
[0087] Prepare E from D:
[0088] Assemble the two-neck flask, magnet, and condenser tube, leaving only the sample injection port in the sealed system, while bubbling the refined 1,4-dioxane to remove the air inside. The raw material compound D (1.00g, 2.05mmol), biboronic acid pinacol ester (0.78g, 3.07mmol), potassium acetate (0.60g, 6.11mmol), 1,1'-bisdiphenylphosphinoferrocene Palladium chloride (0.30 g, 0.41 mmol) was added into the flask, the system was sealed, and the reaction bottle was wrapped with tinfoil. Vacuum blow nitrogen 2-3 times, inject 5 mL of 1,4-dioxane into the reaction flask. Stir and heat the reaction in an oil bath at 90°C for 24 hours. After the reaction, extract the organic layer with dichloromethane, combine the organic phases, dry with anhydrous sodium sulfate, concentrate by rotary evaporation to remove the solvent, and separate and purify by column chromato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com