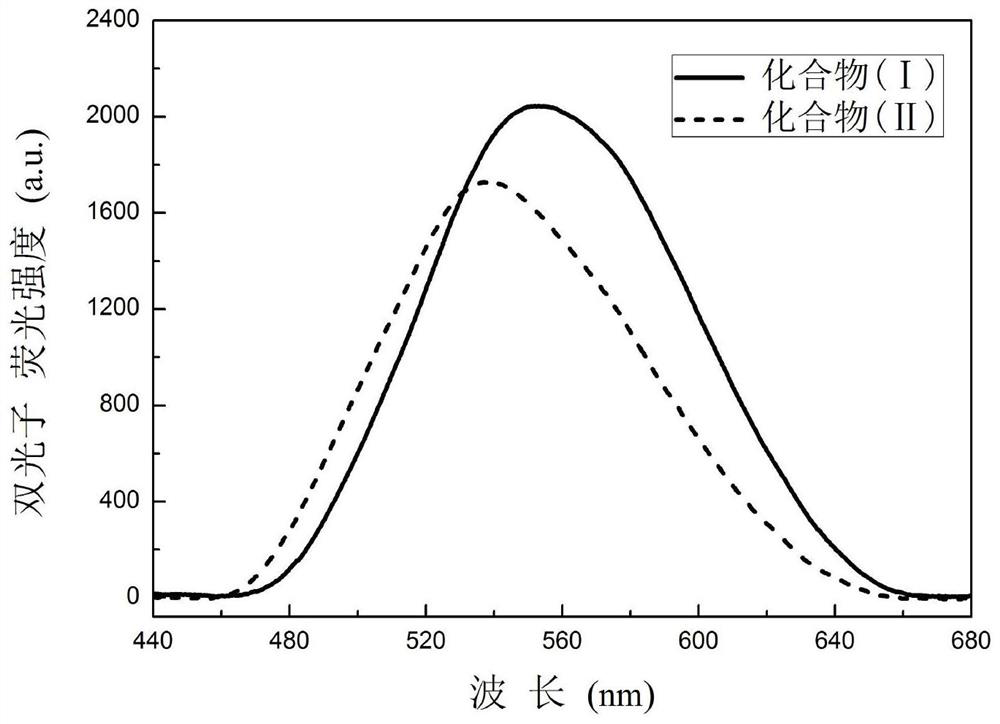
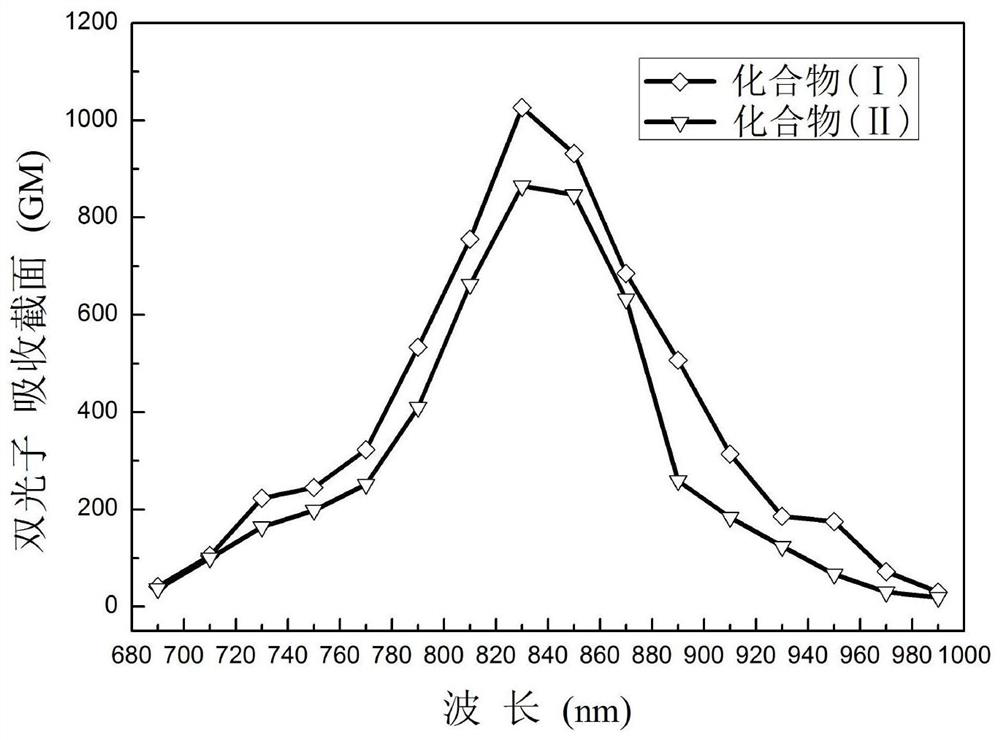
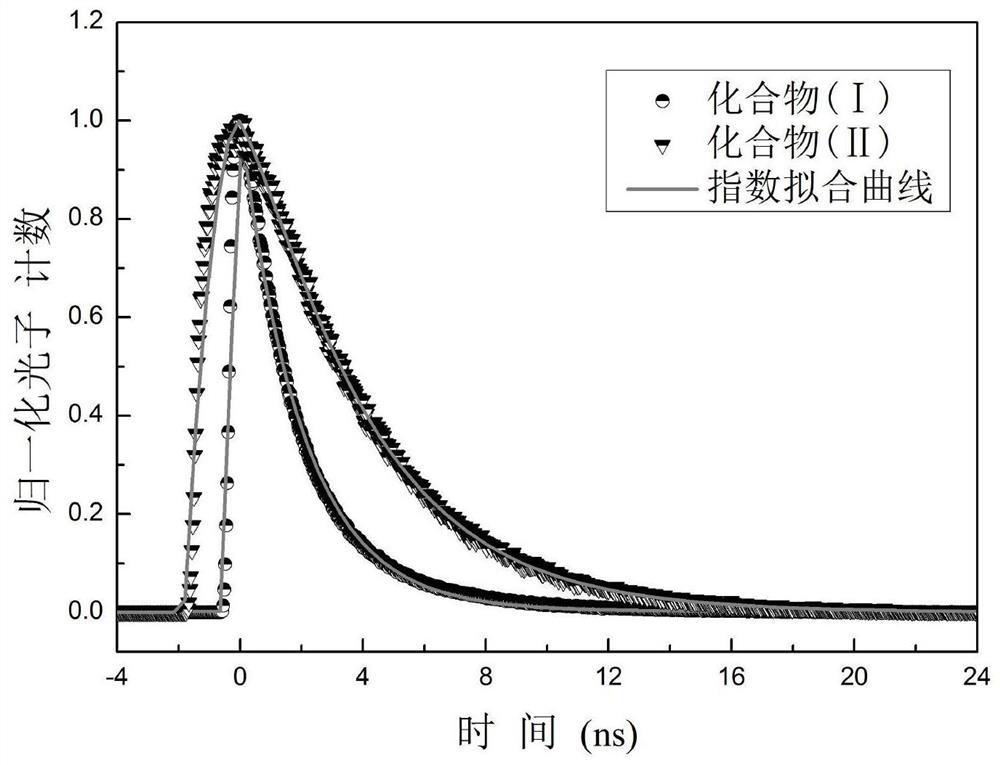
d-π-a-π-d' compound and its synthesis and application
A technology of compound and synthesis method, which is applied in the application fields of high-performance two-photon absorption and two-photon fluorescent materials, and achieves the effect of strong electronegativity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Compound (Ⅲ)
[0038] Add 2.57g (6mmol) of compound (Ⅴ) and 20mL of anhydrous tetrahydrofuran to the reaction flask, and slowly add 0.84g (7.5mmol) of potassium tert-butoxide in an ice bath under the protection of nitrogen to dissolve in 30mL of anhydrous tetrahydrofuran to obtain The solution. After the dropwise addition, a solution obtained by dissolving 1.36g (5mmol) of 4-(9H-carbazol-9-yl)benzaldehyde in 20mL of anhydrous tetrahydrofuran was slowly added dropwise. Then the temperature was raised to room temperature for 8h. Then, 100 mL of distilled water was added to the reaction mixture, followed by extraction with 80 mL of dichloromethane. The obtained organic layer was dried over anhydrous magnesium sulfate, and then separated by silica gel column chromatography [eluent: V (petroleum ether): V (ethyl acetate) = 6:1] to obtain 1.19 g of yellow compound (Ⅲ) . m.p.232-234°C; 1 HNMR (DMSO-d 6 ,500MHz) δ: 8.65(s,1H), 8.27(d,J=7.8Hz,2H), 8.01(d,J=2.8Hz,...
Embodiment 2
[0039] Example 2 Compound (Ⅲ)
[0040] Add 3.21g (7.5mmol) of compound (Ⅴ) and 30mL of anhydrous tetrahydrofuran to the reaction flask, and slowly add 1.51g (13.5mmol) of potassium tert-butoxide in an ice bath under the protection of nitrogen to dissolve in 40mL of anhydrous tetrahydrofuran The resulting solution. After the dropwise addition, a solution obtained by dissolving 1.36g (5mmol) of 4-(9H-carbazol-9-yl)benzaldehyde in 20mL of anhydrous tetrahydrofuran was slowly added dropwise. Then the temperature was raised to room temperature for 16 h. Then, 100 mL of distilled water was added to the reaction mixture, followed by extraction with 80 mL of dichloromethane. The obtained organic layer was dried over anhydrous magnesium sulfate, and then separated by silica gel column chromatography [eluent: V (petroleum ether): V (ethyl acetate) = 6:1] to obtain 1.51 g of yellow compound (Ⅲ) .
Embodiment 3
[0041] Example 3 Compound (IV)
[0042] Add 2.57g (6mmol) of compound (Ⅴ) and 20mL of anhydrous tetrahydrofuran to the reaction flask, and slowly add 0.84g (7.5mmol) of potassium tert-butoxide in an ice bath under the protection of nitrogen to dissolve in 30mL of anhydrous tetrahydrofuran to obtain The solution. After the dropwise addition, a solution obtained by dissolving 0.83g (5mmol) of 3,4-dimethoxybenzaldehyde in 20mL of anhydrous tetrahydrofuran was slowly added dropwise. Then the temperature was raised to room temperature for 8h. Then, 100 mL of distilled water was added to the reaction mixture, followed by extraction with 80 mL of dichloromethane. The obtained organic layer was dried over anhydrous magnesium sulfate, and then separated by silica gel column chromatography [eluent: V (petroleum ether): V (ethyl acetate) = 5:1] to obtain 1.13 g of yellow compound (IV) . m.p.168-170℃; 1 HNMR (DMSO-d 6 ,500MHz) δ: 8.53(s,1H), 7.95(d,J=2.9Hz,1H), 7.67(d,J=16.2Hz,1H), ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com