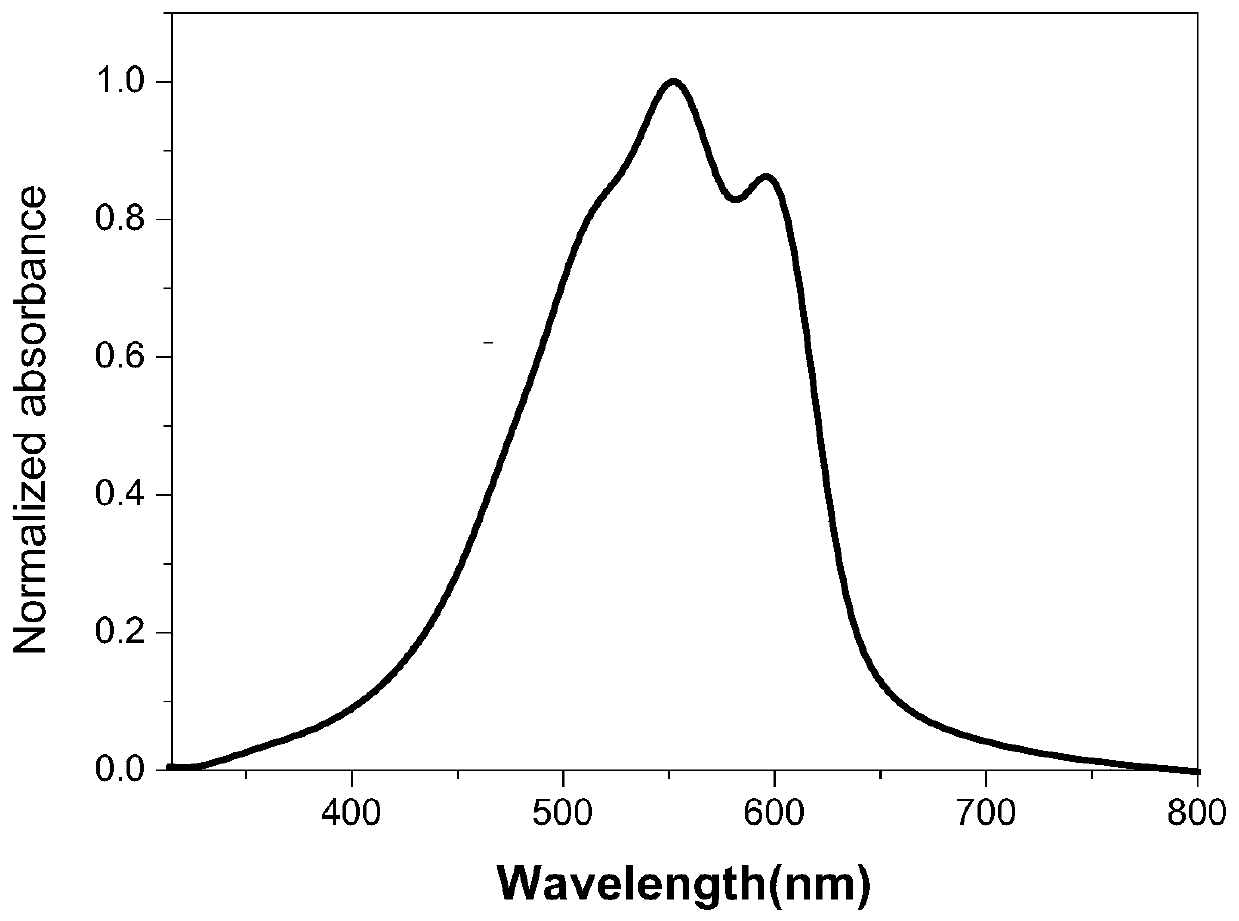
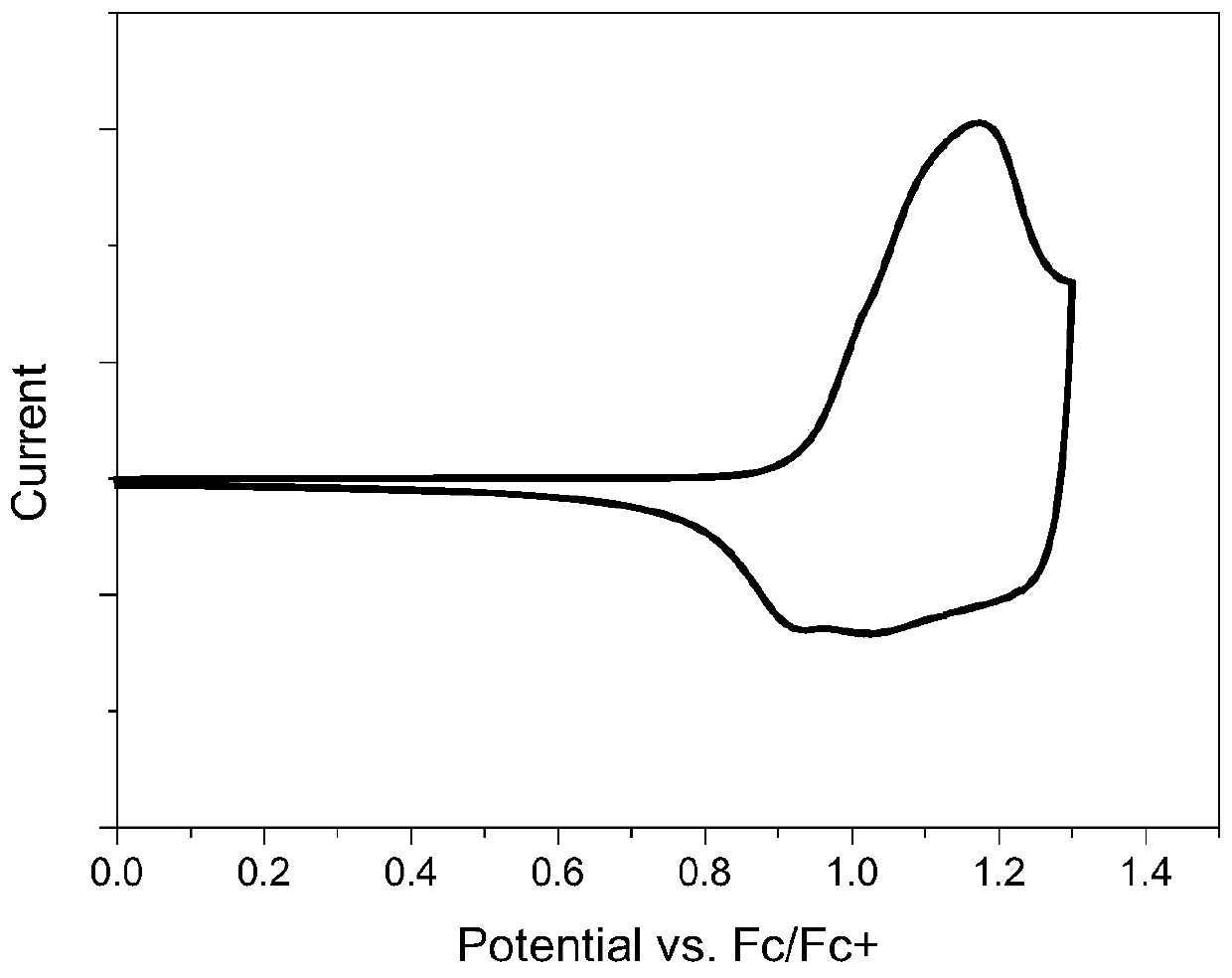
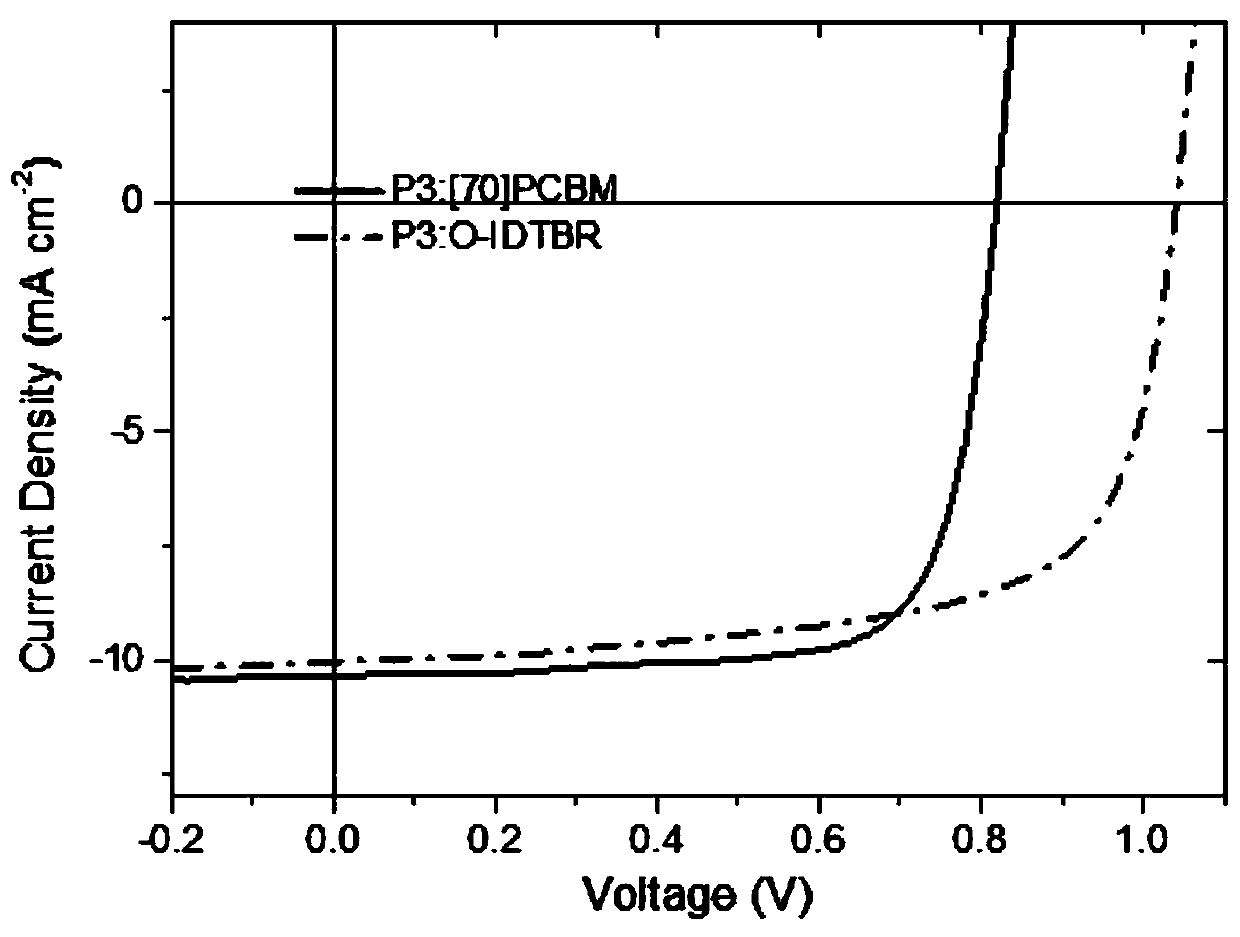
Polythiophene derivative photoelectric material, and preparation method and application thereof
A technology of polythiophene derivatives and optoelectronic materials, applied in circuits, electrical components, electrical solid devices, etc., can solve the problems of low open-circuit voltage short-circuit current, high HOMO energy level, insufficient absorption spectrum, etc., achieving easy synthesis, high Absorption coefficient, satisfying the effect of high-efficiency devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This embodiment provides a monomer M1 and its preparation method.
[0057] The preparation of monomer M1 before polymerization, the reaction formula is as follows:
[0058]
[0059] (1) Dissolve 10mmol of 3-alkylthiophene in 50mL of tetrahydrofuran, add 10mmol of N-bromosuccinimide as a bromination reagent, stir at room temperature for 8h, separate and purify with silica gel chromatography to obtain 2-bromosuccinimide -3-Alkylthiophene.
[0060] (2) 5 mmol of 2-bromo-3-alkylthiophene, 0.5 mmol of bis(cyanobenzene)palladium dichloride, 10 mmol of silver nitrate and 10 mmol of potassium fluoride were added to a 100 mL two-necked flask, After the ventilation was completed, 50 mL of ultra-dry dimethyl sulfoxide was added, and the reaction was heated at 60°C. When the reaction was carried out for 3h and 6h, 10mmol of silver nitrate and potassium fluoride were added respectively, and the reaction was continued for 12h. After the reaction, cool to room temperature, pour ...
Embodiment 2
[0063] This embodiment provides a monomer M2 and its preparation method.
[0064] The preparation of monomer M2 before polymerization, reaction formula is as follows:
[0065]
[0066] (1) Dissolve 10mmol of 3-alkylthiophene in 50mL of N,N-dimethylformamide, add 10mmol of N-bromosuccinimide as a bromination reagent, stir at room temperature for 4h, and use silica gel Separation and purification by chromatographic column to obtain 2-bromo-3-alkylthiophene.
[0067] (2) Add 5mmol of 2-bromo-3-alkylthiophene, 0.05mmol of bis(cyanobenzene)palladium dichloride and 10mmol of silver fluoride into a 100mL two-necked flask, and add 50mL of ultra-dry dimethyl sulfoxide was heated at 80°C for 12h. After the reaction, cool to room temperature, pour the above reaction substance into 100 mL of water, extract three times with dichloromethane, dry the organic phase with anhydrous magnesium sulfate, and separate and purify with silica gel chromatography to obtain the monomer M2.
[0068]...
Embodiment 3
[0070] This embodiment provides a monomer N and its preparation method.
[0071] The preparation of monomer N before polymerization, the reaction formula is as follows:
[0072]
[0073] (1) Carry out Stille coupling reaction with 10mmol of 5,5'-dibromo-2,2'-bithiophene and 24mmol of tributyl(4-alkylthiophen-2-yl)tin, the specific operation is: The two reaction substrates and 0.4 mmol catalyst tetrakis(triphenylphosphine) palladium were added into a 100 mL two-necked flask, and 50 mL of distilled toluene was added as the reaction solvent after the ventilation was completed, and the reaction was heated under reflux for 12 h. After the reaction was completed, it was cooled to room temperature, and the above reaction substance was poured into 100 mL of water, extracted three times with dichloromethane, and the organic phase was dried with anhydrous magnesium sulfate, separated and purified by silica gel chromatography.
[0074] (2) The product obtained in the first step is br...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com