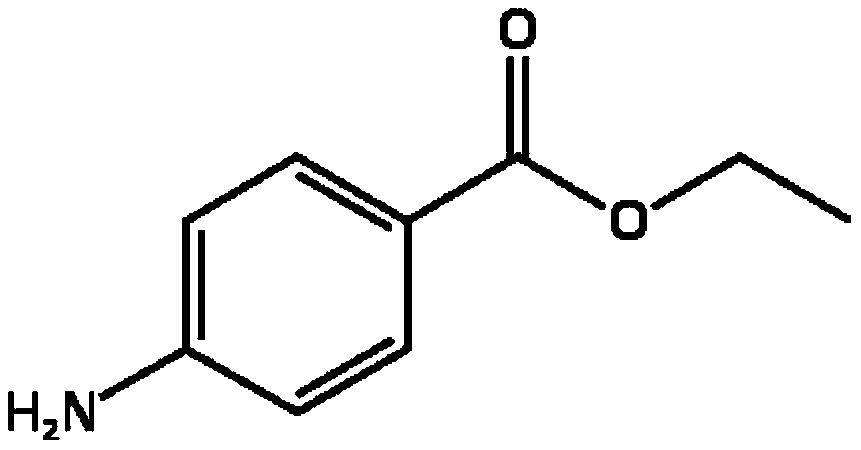
Pharmaceutical composition comprising benzocaine with enhanced stability
A technology of benzocaine and stabilizer, applied in the field of pharmaceutical compositions, can solve the problems such as undesired synthesis of benzocaine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Embodiment 1: preparation preparation
[0069] Four different tablet formulations were produced using the following ingredients:
[0070] Element
[g] Ingredients per 1000g
Sodium carboxymethyl cellulose
10.0
peppermint
5.0
Povidone K 25
10.0
20.0
Sodium Saccharinx 2 H2O
1.0
79.9
L-Lysine Monohydrate
0 / 1.5 / 3.0 / 10.0
872.7 / 870.7 / 869,2.7 / 859.2
Benzocaine
1.9
sum
1000g
[0071] Tablets of 1000 mg each are provided with the stated ingredients using the following method:
[0072] Sodium saccharin, benzocaine and povidone K25 were dissolved in 96% ethanol at room temperature to obtain drug solution 1.
[0073] Drug solution 1 was sprayed onto about 50% sorbitol in a fluidized bed system, and dried at 25-35° C. until ethanol evaporated to obtain dry material 1 .
[0074] The dry mass 1 was mixed with the remaining appro...
Embodiment 2
[0078] Embodiment 2: Analytical method
[0079] Analysis was performed using high pressure liquid chromatography (HPLC) according to Ph. Eur. 2.2.29.
[0080] The separation column is Kinetex C18, 150mm x 4.6mm. Eluent A was 20 mM sodium acetate in water, pH adjusted to 4.7 with acetic acid. Eluent B is acetonitrile. The applied gradient starts at 14 vol.-% eluent B and ends at 80 vol.% eluent B at a rate of about 1 ml / min. The injection volume was 15 μl.
[0081] Analysis was performed at 8°C by UV detection at 288nm and reference 400nm.
[0082] 0.5 g of the tablet of Example 1 was thoroughly dissolved in 50 ml of 25% acetonitrile. The control was a solution of 1.5 mg benzocaine in 100 ml 25% acetonitrile.
[0083] This control showed a peak at an elution volume of approximately 19 ml, which was determined to be undegraded benzocaine. Samples stored for 1 month showed additional peaks (B1 to B12) between elution volumes of 6.5 ml and 13 ml, with a distinct peak at 7.5...
Embodiment 3
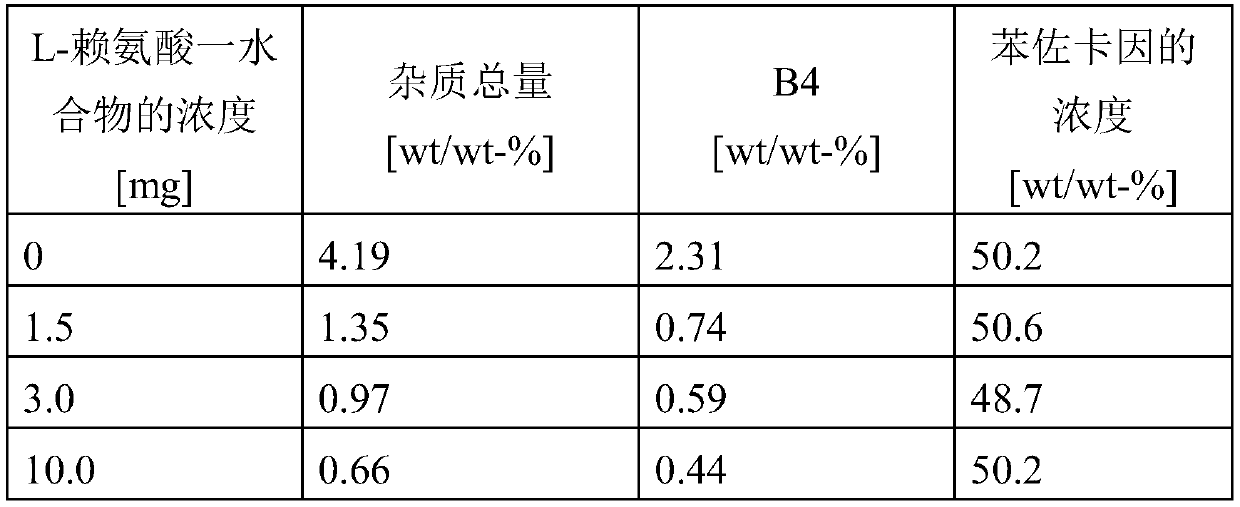
[0084] Example 3: Results
[0085]
[0086] It was found that the presence of L-lysine monohydrate in a quantitative manner prevented the formation of impurities B1 to B12, especially of impurity B4, which was thought to represent the Maillard product of benzocaine with glucose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com