Azulene-based modified polystyrene compound and application thereof
A polystyrene and compound technology, applied in the direction of analysis by chemical reaction of materials, organic chemistry, material analysis by observing the influence of chemical indicators, etc., can solve the lack of regular understanding, synthesis and molecular design difficulties , less organic optoelectronic materials, etc., to achieve significant aromaticity, good reversible responsiveness, and the effect of facilitating purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
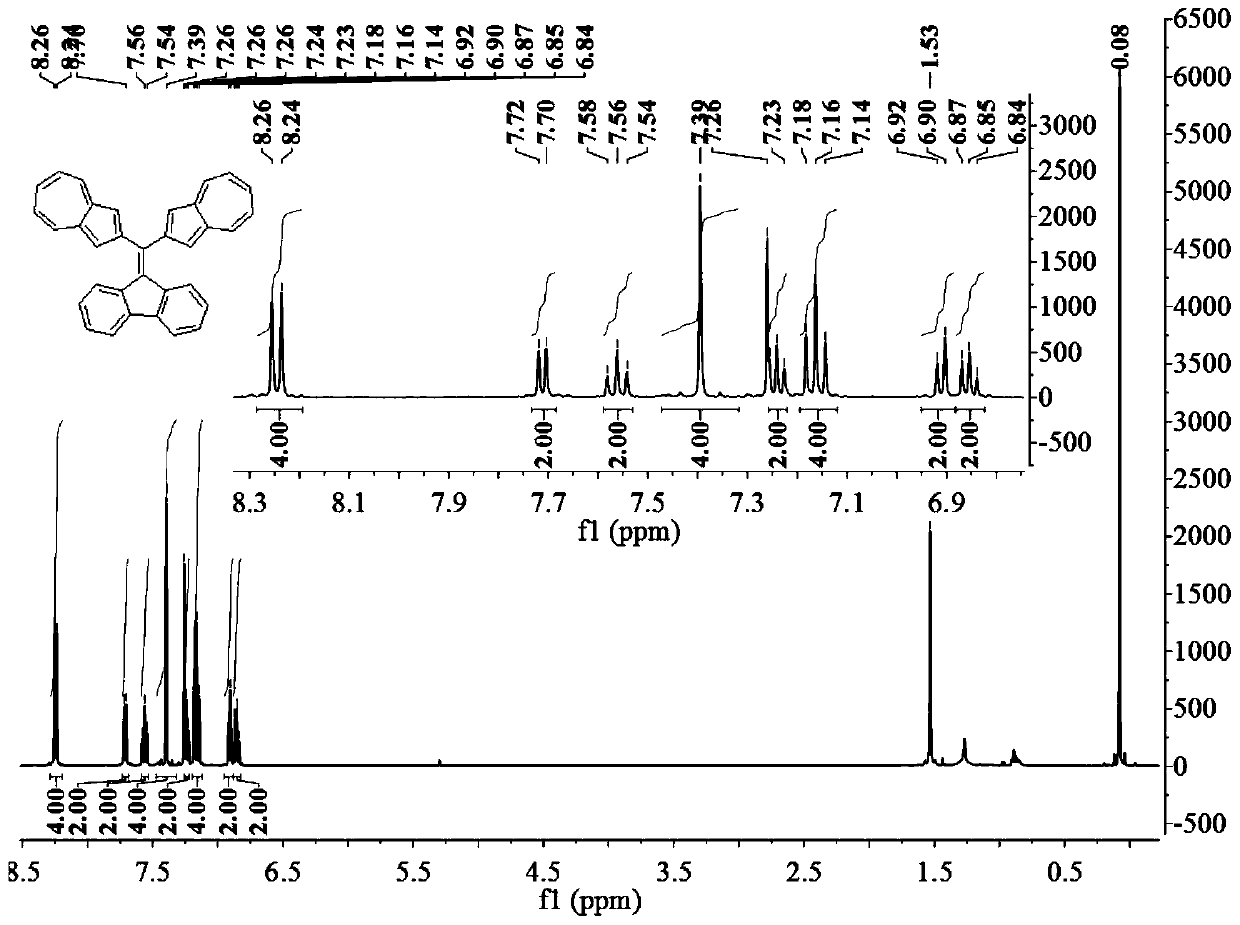
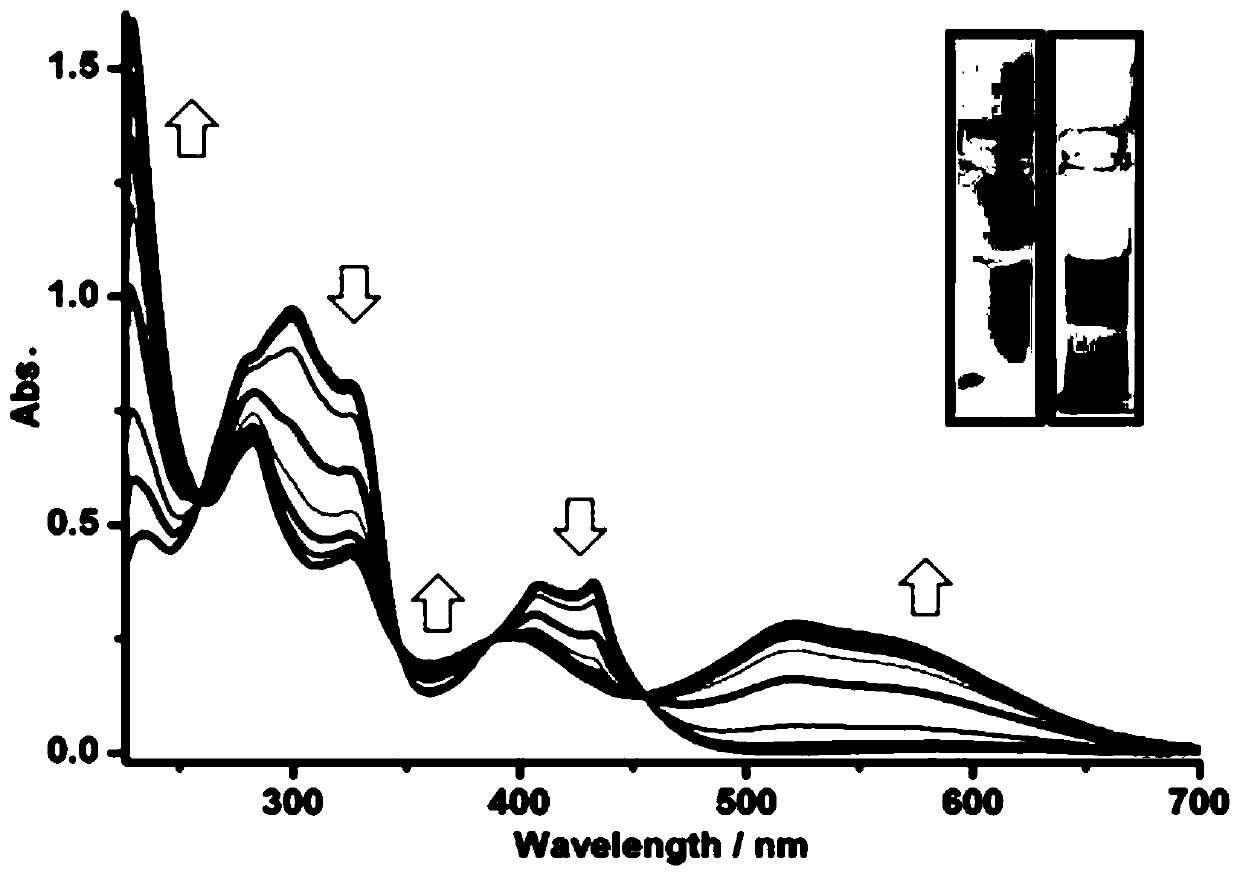
[0028] Weigh 0.25g of 2-iodoazulene (1.00mmol), put it into a 50mL round bottom flask together with 30ml of tetrahydrofuran and 5ml of water, then add 0.36g of (1,2,2-triphenyl Vinyl) boronic acid (1.20mmol), 0.39g cesium carbonate (1.20mmol) and 0.06g catalyst tetrakis (triphenylphosphine) palladium (0.05mmol), degassed for 0.5 hours and heated to reflux under argon protection, slowly stirred the reaction In 24 hours, the molar ratio of 2-iodoazulene, (1,2,2-tristyryl)boronic acid, and cesium carbonate was 10:12:12. After the reaction was completed, it was cooled to room temperature, the mixture was extracted three times with 50 ml of chloroform, and the organic phases were combined. Then washed with brine, anhydrous Na 2 SO 4 Dry and filter. After removing the solvent using a rotary evaporator, using a mixture of hexane and dichloromethane at a volume ratio of 2:1 as the eluent, 0.33 g of a dark blue solid was obtained by silica gel column chromatography, which was 2-(1,2...
Embodiment 2
[0038] Similar to Example 1, except that (1,2,2-tristyryl)boronic acid is replaced by 4-(1,2,2-tristyryl)phenylboronic acid. Weigh 0.25g of 2-iodoazulene (1.00mmol), put it into a 50mL round bottom flask together with 30ml of tetrahydrofuran and 5ml of water, and then add 0.45g of 4-(1,2,2-triphenylethylene ) Phenylboronic acid (1.20mmol), 0.39g cesium carbonate (1.20mmol) and 0.06g catalyst tetrakis (triphenylphosphine) palladium (0.05mmol), degassed for 0.5 hour and heated to reflux under argon protection, slowly stirred the reaction In 24 hours, the molar ratio of 2-iodoazulene, 4-(1,2,2-triphenylethylene)phenylboronic acid, and cesium carbonate was 10:12:12. After the reaction was completed, it was cooled to room temperature, the mixture was extracted three times with 50 ml of chloroform, and the organic phases were combined. Then washed with brine, anhydrous Na 2 SO 4 Dry and filter. After using a rotary evaporator to remove the solvent, a mixture of hexane and methyl...
Embodiment 3
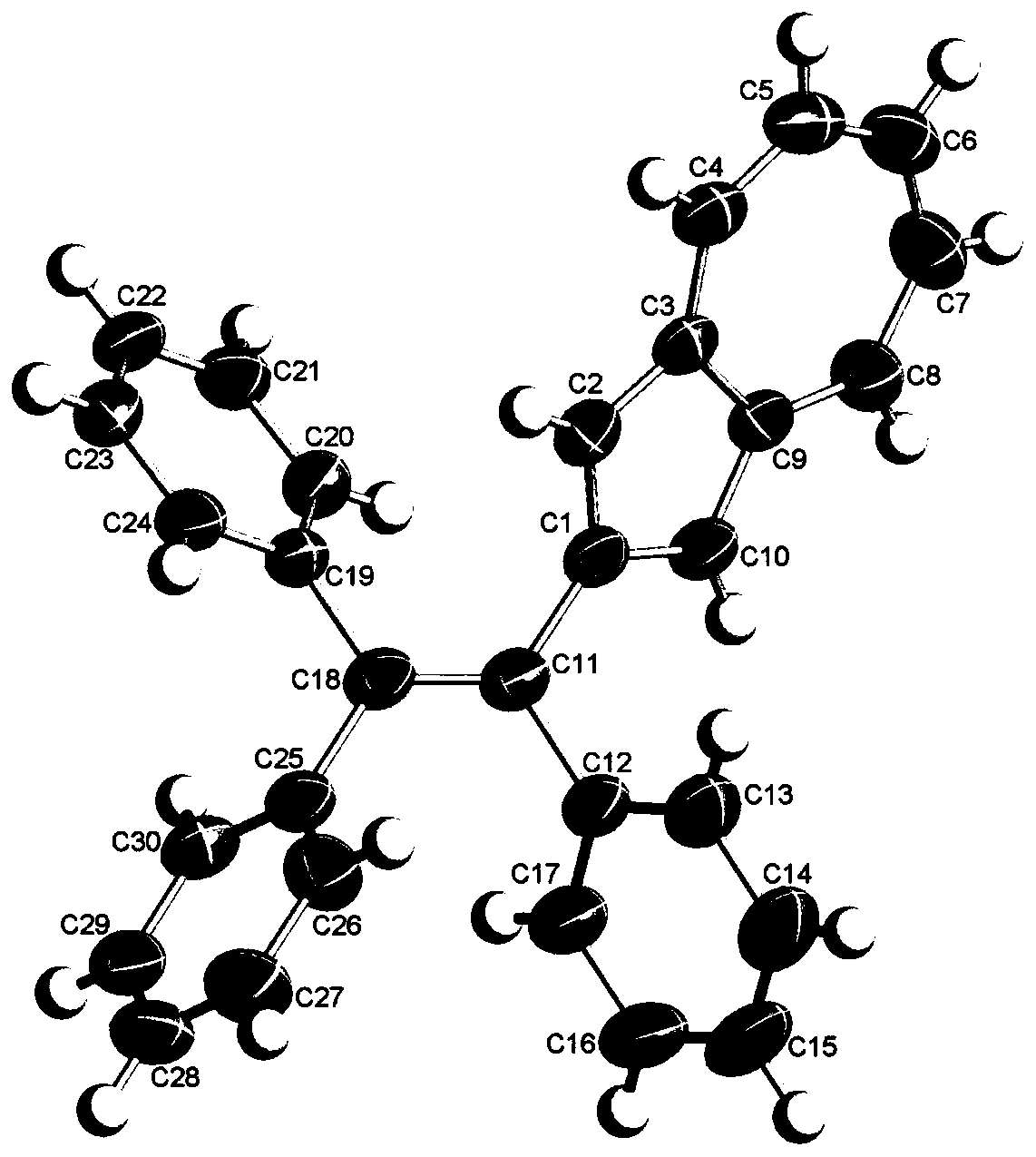
[0042] Weigh 0.34g of 1,1-dibromo-2,2-diphenylethylene (1.00mmol), put it into a 50mL round bottom flask together with 50ml of tetrahydrofuran and 5ml of water, and then add 0.51g of 2-(2-Azulenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Azu-Bpin) (2.20mmol), 0.98g cesium carbonate (3.00 mmol) and 0.12g catalyst tetrakis(triphenylphosphine) palladium (0.10mmol), heated to reflux under the protection of argon, and stirred slowly for 48 hours, 1,1-dibromo-2,2-diphenylethylene, 2 The molar ratio of -(2-azulene)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (Azu-Bpin) and cesium carbonate is 5:11 :15. When the reaction is over, cool to room temperature, the mixture is extracted three times with 50ml chloroform, the organic phases are combined, then washed with brine, anhydrous Na 2 SO 4 Dry and filter. After the solvent was removed by a rotary evaporator, 0.34 g of a green solid was separated by silica gel column chromatography with chloroform as an eluent, and the yield was 78%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com