Highly-soluble high-performance semiconductor conjugated polymer and synthetic method thereof
A conjugated polymer, high solubility technology, applied in semiconductor devices, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve the research and application of high-performance semiconductor conjugated polymers, high-performance semiconductor conjugated polymerization Less matter, affecting human health and other issues, to achieve the effect of improving charge transport performance, improving solubility, and improving electrical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
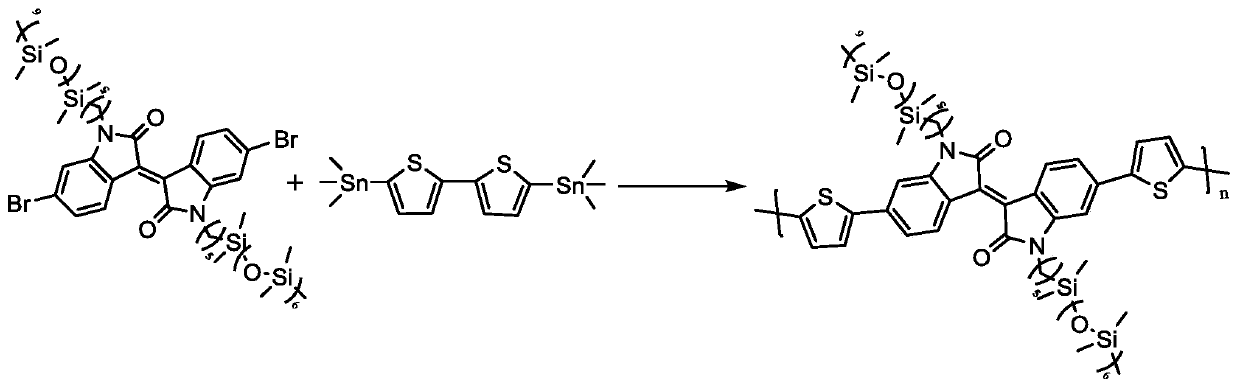
[0030] Embodiment 1, synthetic polymer PIID-C 5 -Si 7
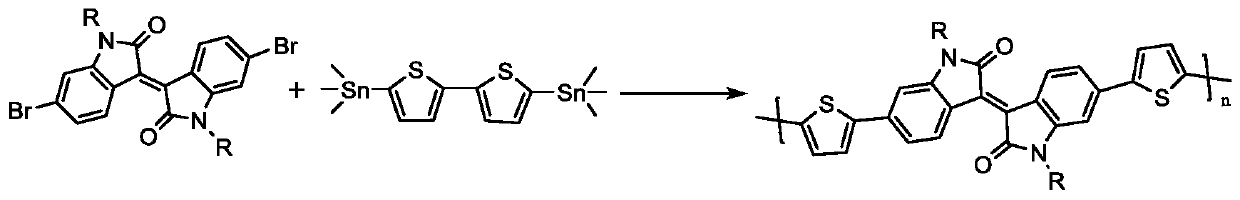
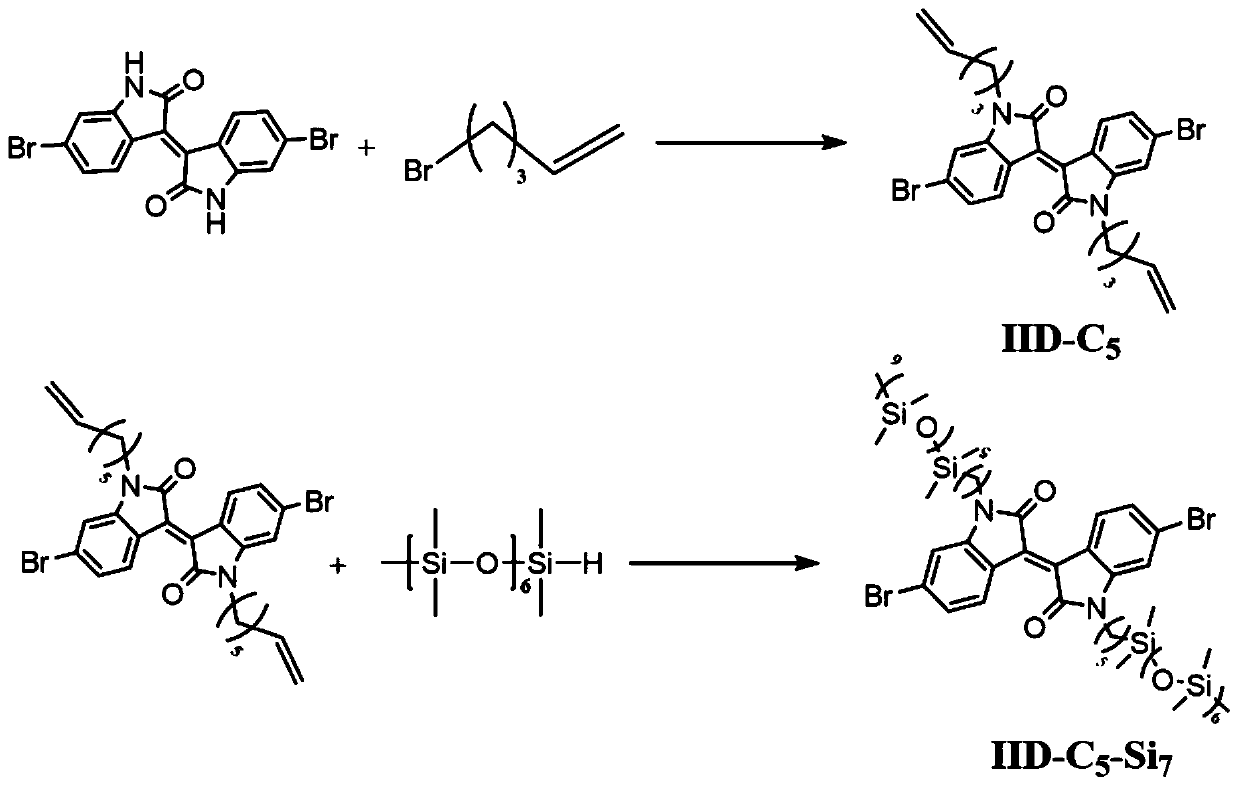
[0031] like figure 2 Shown, the isoindigo dibromomonomer IID-C used in this embodiment 5 -Si 7 The synthesis method is as follows:
[0032] Sodium hydrogen gas (7.4 mmol) was added to a solution of 6,6'-dibromoisoindigo (3.1 mmol) in anhydrous DMF (25 mL) under ice bath condition. After 20 minutes, 5-bromo-1-pentene (7.4 mmol) was injected through a septum under nitrogen. The mixture was poured into water (200 mL) and stirred at room temperature for 3 hours. use CH 2 Cl 2 The organic phase was extracted, washed with brine, and washed with MgSO 4 dry. The crude product was washed three times with methanol (100 mL) to give the product IID-C as a dark red solid 5 .
[0033] Under the protection of nitrogen, the compound IID-C 5 (1.8mmol) was dissolved in anhydrous toluene (20mL). Pentamethylsilylhydrogen (4.4 mmol) was injected via a sterile syringe, followed by the addition of a drop (50 μL) of Karstedt's cat...
Embodiment 2
[0036] Embodiment 2, synthetic polymer PIID-C 6 -Si 7
[0037]The isoindigo dibromomonomer IID-C used in this embodiment 6 -Si 7 The synthesis method is as follows:
[0038] Sodium hydrogen gas (7.4 mmol) was added to a solution of 6,6'-dibromoisoindigo (3.1 mmol) in anhydrous DMF (25 mL) under ice bath condition. After 20 minutes, 6-bromo-1-hexene (7.4 mmol) was injected through a septum under nitrogen. The mixture was poured into water (200 mL) and stirred at room temperature for 3 hours. use CH 2 Cl 2 The organic phase was extracted, washed with brine, and washed with MgSO 4 dry. The crude product was washed three times with methanol (100 mL) to give the product IID-C as a dark red solid 6 .
[0039] Under the protection of nitrogen, the compound IID-C 6 (1.8mmol) was dissolved in anhydrous toluene (20mL). Pentamethylsilylhydrogen (4.4 mmol) was injected via a sterile syringe, followed by the addition of a drop (50 μL) of Karstedt's catalyst (platinum divinylte...
Embodiment 3
[0042] Embodiment 3, synthetic polymer PIID-C 7 -Si 7
[0043] The isoindigo dibromomonomer IID-C used in this embodiment 7 -Si 7 The synthesis method is as follows:
[0044] Sodium hydrogen gas (7.4 mmol) was added to a solution of 6,6'-dibromoisoindigo (3.1 mmol) in anhydrous DMF (25 mL) under ice bath condition. After 20 minutes, 7-bromo-1-heptene (7.4 mmol) was injected through a septum under nitrogen. The mixture was poured into water (200 mL) and stirred at room temperature for 3 hours. use CH 2 Cl 2 The organic phase was extracted, washed with brine, and washed with MgSO 4 dry. The crude product was washed three times with methanol (100 mL) to give the product IID-C as a dark red solid 7 .
[0045] Under the protection of nitrogen, the compound IID-C 6 (1.8mmol) was dissolved in anhydrous toluene (20mL). Pentamethylsilylhydrogen (4.4 mmol) was injected via a sterile syringe, followed by the addition of a drop (50 μL) of Karstedt's catalyst (platinum divinyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com