Cortex moutan formula granule standard substance preparation and quality control method
A quality control method and formula granule technology, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, measuring devices, etc., can solve the problems of lack of a reasonable comparison, and achieve quality control, scientific production, and quality assurance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of the formula granules for the reference substance of Cortex Moutan:
[0027] (1) Weigh the raw materials: 3400g of Moutan Bark pieces;
[0028] (2) Put the decoction pieces of Moutan bark into the decocting pot, decoc two times, add 9 times the amount of water for the first time, soak for 30 minutes, decoct for 30 minutes, add 7 times the amount of water for the second time, and cook for 30 Minutes, the medicinal solution is filtered, the filtrate is combined, and the volatile oil is steam distilled for 2 hours. The distilled liquid is collected for use; the medicinal solution is pumped into the concentration tank, concentrated (vacuum -0.090-0.099MPa), concentrated to a relative density of 1.07-1.09 65°C) clear ointment, spare;
[0029] (3) Place the clear paste obtained in step 2 in a stainless steel tray of a certain specification, and place it in a freeze dryer, close the door and turn on the refrigeration, and wait for the temperature of the partition to dr...
Embodiment 2
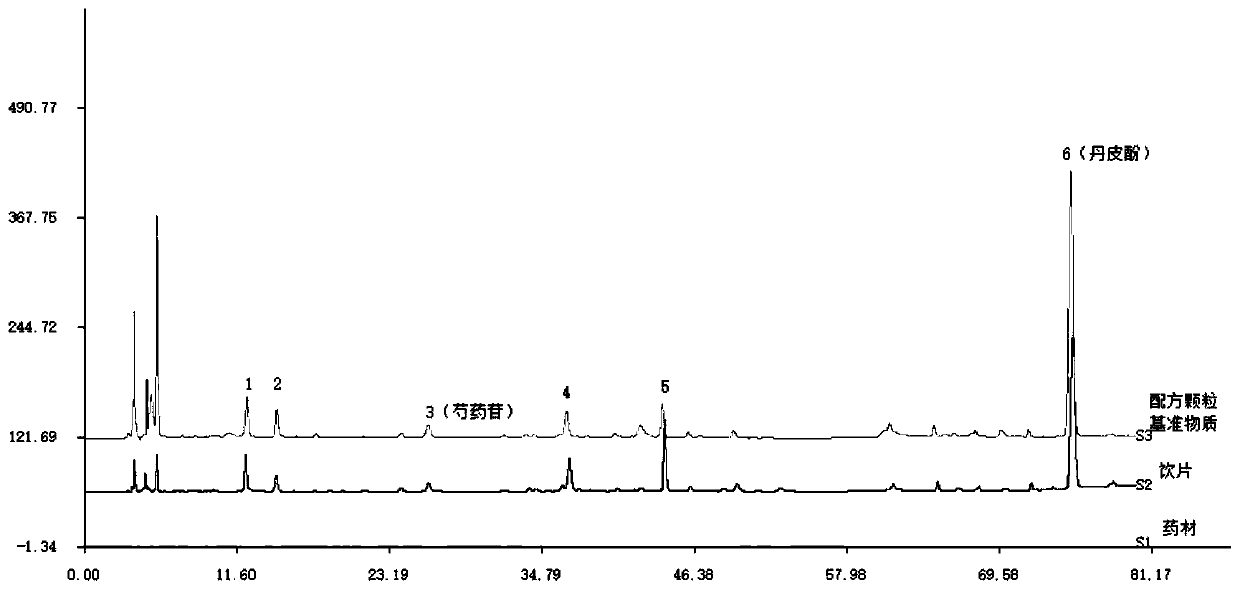
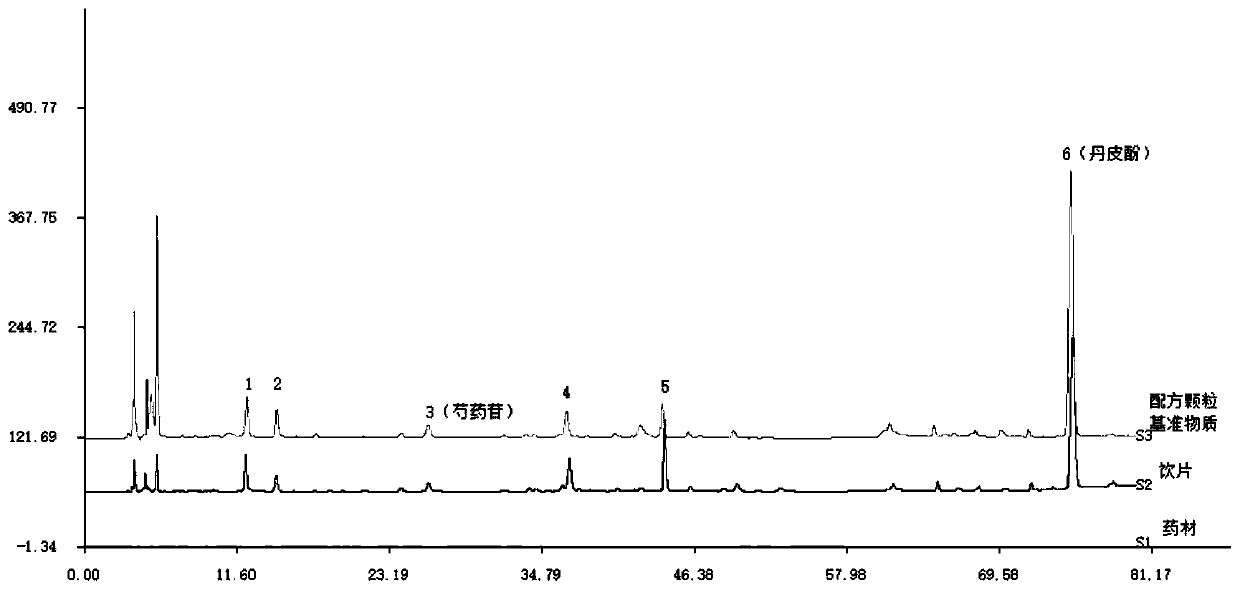
[0035] The quality control method of the HPLC characteristic map of the reference substance of Moutan bark formula granules:
[0036] (1) Take 0.2g of this product powder, medicinal powder, and decoction pieces powder, respectively, accurately weigh them, place them in a 25ml measuring flask, add 20ml of methanol, ultrasound for 30min, let stand to cool, set the volume, filter, and use the filtrate as the test product;
[0037] (2) Take 0.6mg of paeoniflorin, place it in a 10ml volumetric flask, dissolve it with methanol and dilute to the mark, as a reference; take 1.0mg of paeonol, place it in a 10ml volumetric flask, dissolve it with methanol and dilute to volume Scale, as a reference;
[0038] (3) Refer to "Chinese Pharmacopoeia" 2015 Edition Four General Rules 0512 High Performance Liquid Chromatography test, respectively accurately absorb 5-10μl of the test solution and control solution, inject into the chromatograph, and determine according to the following chromatographic cond...
Embodiment 3
[0045] Determination and determination of moisture, extract and content in the reference material of Moutan bark formula granules:
[0046] The moisture, extract and content of the three batches of Paeonia Suffruticosa formula granule reference materials prepared by the above method were tested. The moisture is tested according to conventional methods. The extract is determined in accordance with the hot dipping method under the alcohol-soluble extract determination method (Chinese Pharmacopoeia 2015 Edition Sibu General Principle 2201), using ethanol as the solvent. The content determination is based on the determination method under the item of Cortex Moutan ("Chinese Pharmacopoeia" 2015 Edition One). The inspection results are shown in Table 3, indicating that all the indicators meet the standards.
[0047] Table 3 Test results
[0048] batch number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com