Pyrazine compound and application thereof
A compound and halogen technology, which is applied to pyrazine compounds and their application in lithium isotope extraction and separation, can solve the problems of difficult synthesis of extractants, pollution, and low extraction and separation coefficients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
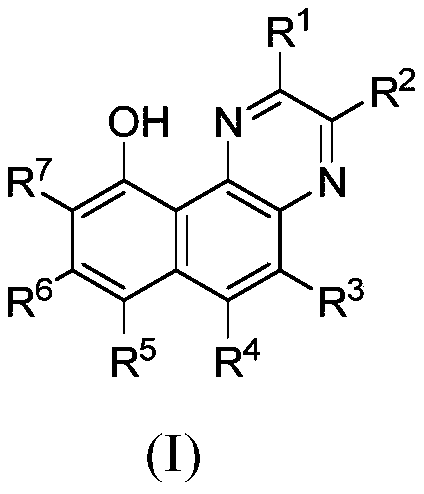
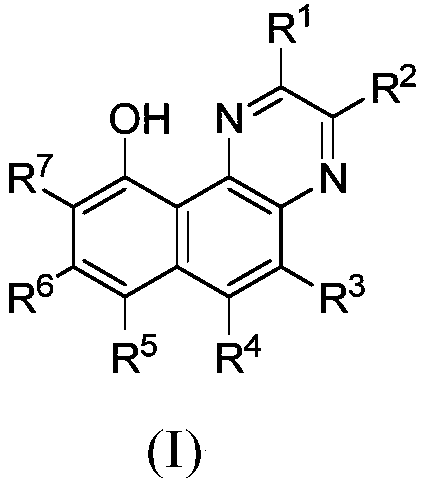
[0087] The preparation of formula (I) compound
[0088] The compounds of the present invention can be prepared by standard methods known in the art or by known methods analogous thereto. General methods for preparing the compounds of the invention are set forth below. The starting materials and reagents described in the following experimental reaction schemes are generally commercially available. A preparation method of a compound of formula I, comprising the steps of:
[0089]
[0090] (1) In the first solvent, the compound of formula A reacts with the compound of formula D to obtain the compound of formula B;
[0091] (2) In the second solvent, in the presence of palladium acetate and iodobenzene acetate, the compound of formula B undergoes an oxidation reaction to obtain the compound of formula (I);
[0092] The definition of each group is as described in the first aspect of the present invention.
[0093] In another preferred embodiment, the cyclization reaction has o...
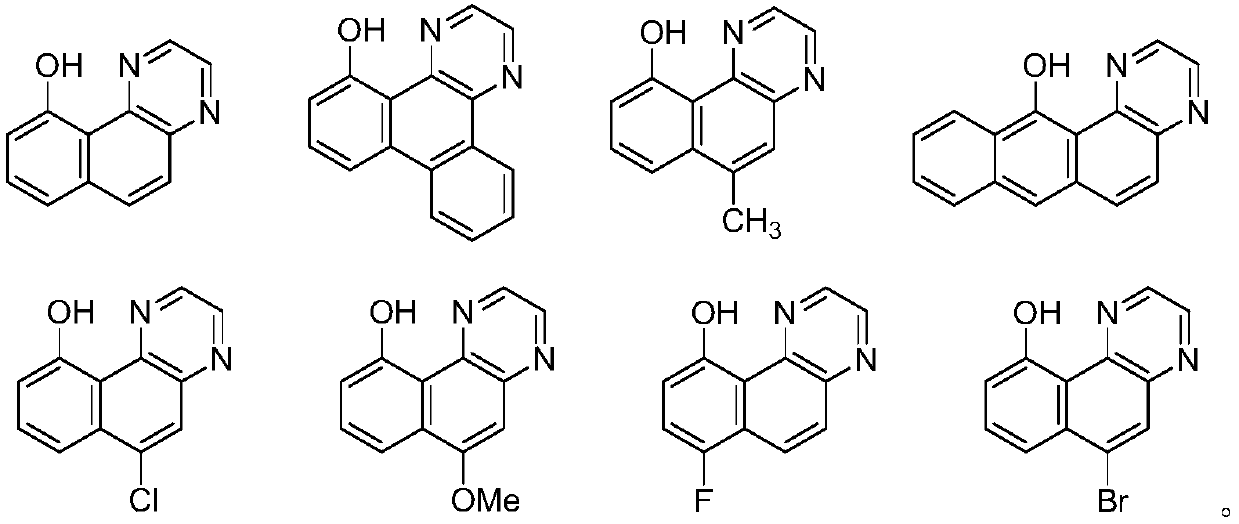
Embodiment 1
[0151] The synthetic method of compound C1:
[0152]
[0153] Step 1: Add 0.158g (1mmol) of commercially available compound A1 and 5mL of ethanol to the three-neck flask, add dropwise a solution of 0.07mL of ethylenediamine and 1ml of ethanol at room temperature, stir for 15min and heat to 100°C , Reaction 4h. Add water, extract with ethyl acetate to obtain an organic layer, wash with saturated brine, dry the organic layer with anhydrous magnesium sulfate, and obtain 0.076 g of compound B1 by column chromatography, with a yield of 42%.
[0154] The second step: add 0.576g of compound B1 (3.2mmol), 2.254g of iodobenzene acetate (7mmol), 0.030g of palladium acetate (0.125mmol) in the sealed tube, and add 30mL of acetonitrile and 1mL of acetic anhydride, mix and heat to 165°C and stirred for 36h. Evaporate acetonitrile, add 1.60g of sodium hydroxide and 30mL of methanol, hydrolyze for 4h, neutralize, extract with ethyl acetate to obtain an organic layer, wash with saturated ...
Embodiment 2
[0156] The synthetic method of compound C2:
[0157]
[0158] Step 1: Add 1.04g (A2, 5mmol) of commercially available raw materials 9,10-phenanthrenequinone and 20mL of absolute ethanol to a 100mL one-mouth bottle, add about 0.35mL (5mmol) of ethylenediamine and 5mL of anhydrous Mixed solution of ethanol; the reaction solution is a yellow turbid solution. After heating up to 100°C for about 2 hours, solids precipitate out. Stop stirring for about 4 hours, filter to obtain a light yellow product, stir and filter with ethanol and a little dichloromethane, and obtain 0.805 The product compound B2 of g, yield 70%.
[0159] Step 2: Add 0.231g of compound B2 (1.0mmol), 0.676g of iodobenzene acetate (2.0mmol), 0.010g of palladium acetate (0.042mmol) into the sealed tube, and add 5mL of dry acetonitrile, mix and heat to 165°C And stirred the reaction for 18h. Acetonitrile was evaporated, sodium hydroxide and methanol were added, hydrolyzed, neutralized, extracted with ethyl aceta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com