Phenylethanolamine-based beta receptor agonist synthesis method
A technology of phenylethanolamine and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of aminohydroxy compounds, etc., can solve the problems of complicated operation, high cost, low atom utilization rate, etc., and achieves cheap and easy-to-obtain raw materials, The effect of high atom utilization, simple and efficient synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
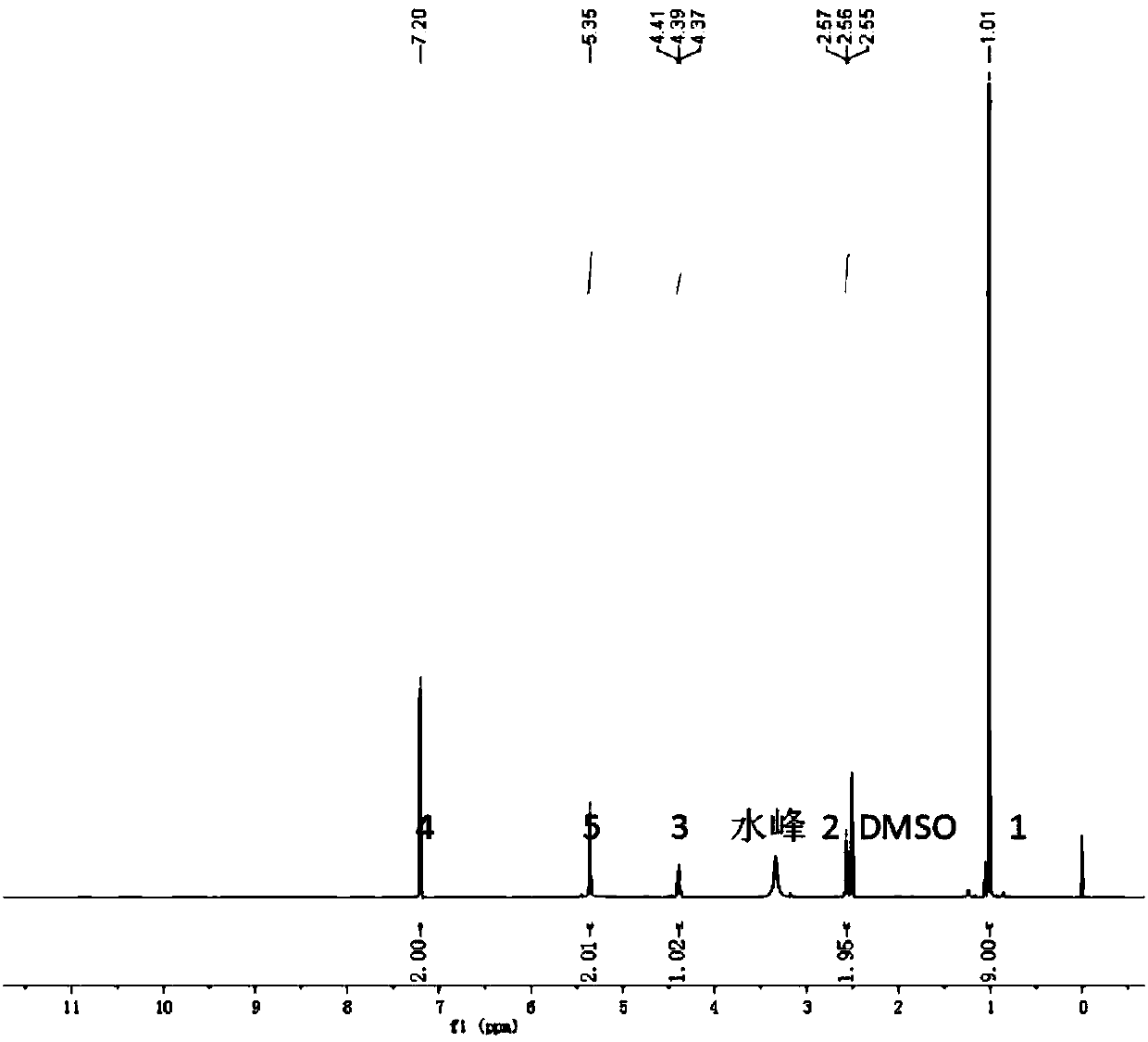
[0039] The synthetic method of 4-amino-3,5-dichloroacetophenone (Clenbuterol)
[0040]
[0041] Weigh 7.9g (59.2mmol) NCS in a 250ml three-neck flask, add 60ml MeCN, stir to dissolve, and slowly drop 4-aminoacetophenone (4g, 29.6mmol) dissolved in 50ml acetonitrile into the reaction with a constant pressure dropping funnel device, the dropwise addition was completed in half an hour, and stirred at room temperature for 3 hours. Rotary evaporate most of the solvent, add ethyl acetate and stir to dissolve, wash with 50ml pure water 3 times each time, collect the organic phase, and dry over anhydrous sodium sulfate. After filtration, the solvent was spin-dried to obtain 4.8 g of the main product acetophenone intermediate 4-amino-3,5-dichloroacetophenone, and the yield was 80%; at the same time, 0.9 g of by-product 4-amino-3-chloroacetophenone was obtained. g, the productive rate is 18%, which can be used in Example 5 as the raw material for the preparation of bromoclobuterol. ...
Embodiment 2
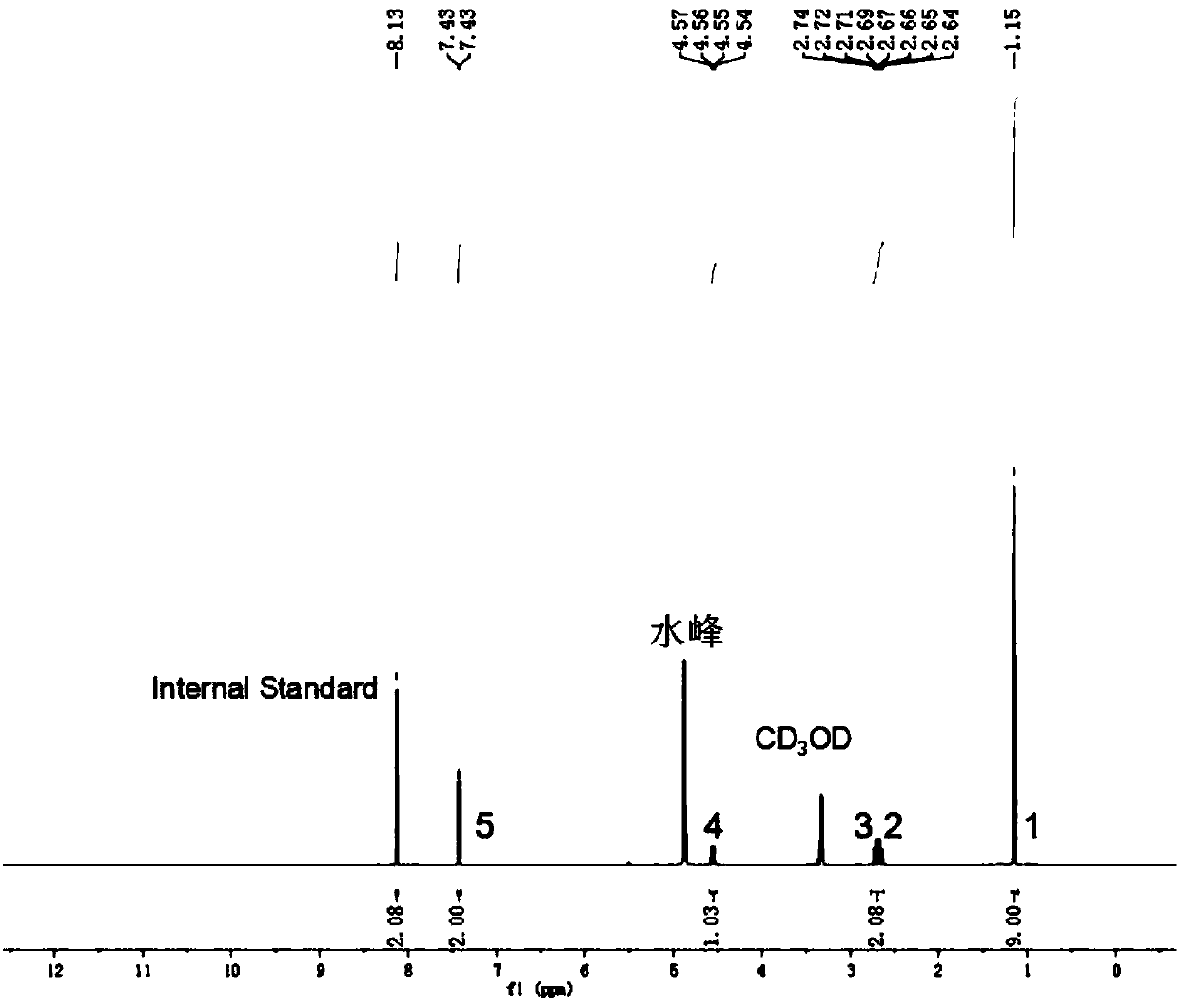
[0052] Synthetic method of 4-amino-3,5-dibromoacetophenone (bromobuterol)
[0053]
[0054] Weigh 11.6g, 65.1mmol N-bromosuccinimide (NBS) in a 250ml three-necked flask, add 60mlMeCN, stir to dissolve, and use a constant pressure dropping funnel to dissolve 4g, 29.6mmol 4-amino Acetophenone was slowly dropped into the reaction device, and the dropwise addition was completed in half an hour, and stirred at room temperature for 3 hours. Rotary evaporate most of the solvent, add ethyl acetate and stir to dissolve, wash 3 times with 50ml pure water, collect the organic phase, and dry over anhydrous sodium sulfate. After filtration, the solvent was spin-dried to obtain 6.9g of acetophenone intermediate main product 4-amino-3,5-dibromoacetophenone, and the yield was 80%; at the same time, 1.2g of by-product 4-amino-3-bromoacetophenone was obtained Ketones, with a yield of 20%, can be used in Examples 3 and 4 as raw materials for the preparation of sibuterol and cimaterol.
[00...
Embodiment 3
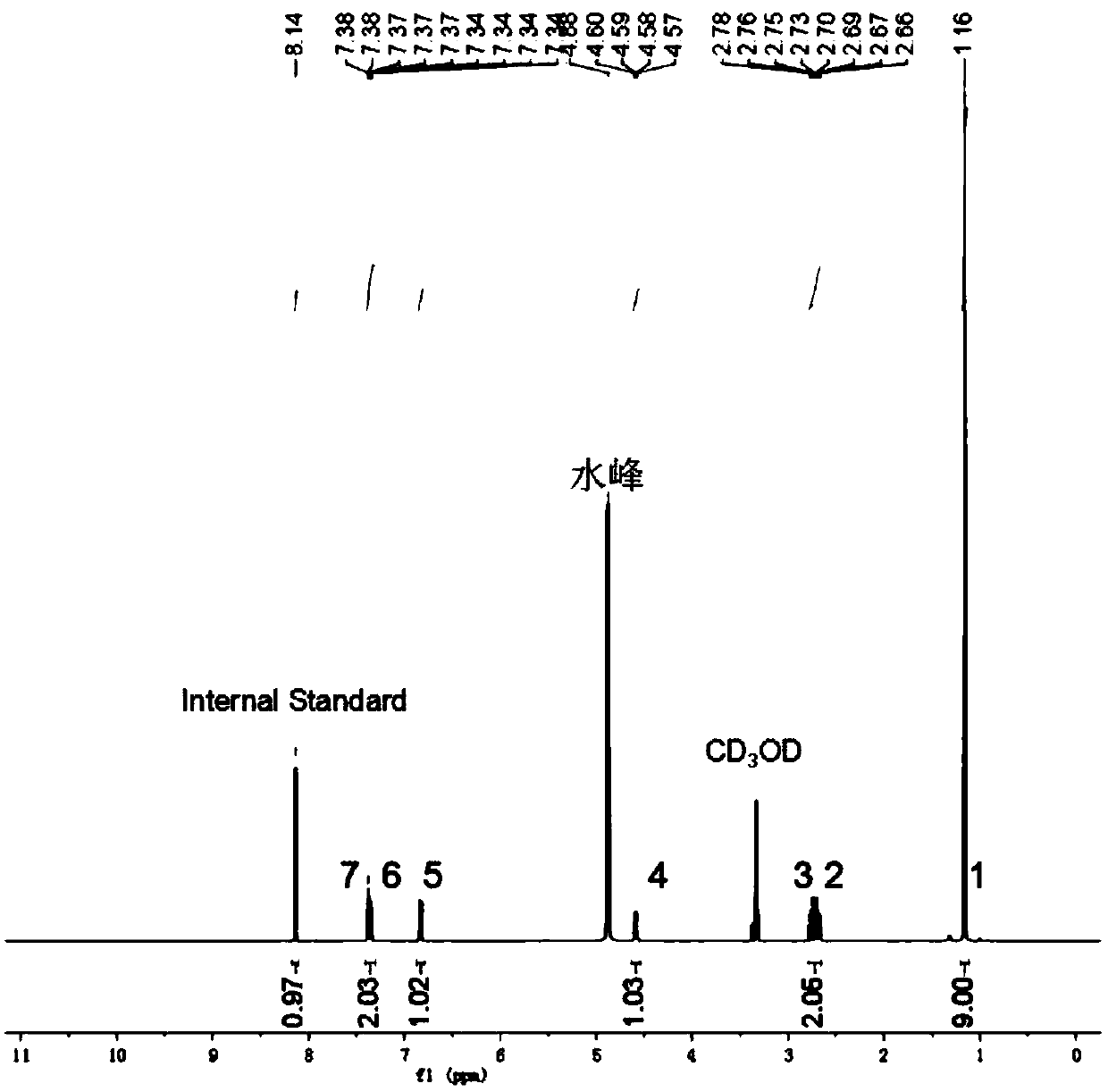
[0066] The synthetic method of 4-amino-3-bromoacetophenone (sibuterol)
[0067]
[0068] Weigh 4g (29.6mmol) of 4-aminoacetophenone into a 250ml three-neck flask, add 40ml of MeCN, stir to dissolve, and slowly drop 5.8g (32.6mmol) of NBS dissolved in 50ml of acetonitrile into the reaction device with a constant pressure dropping funnel , The dropwise addition was completed in half an hour, and stirred at room temperature for 3 hours. Rotary evaporate most of the solvent, add ethyl acetate and stir to dissolve, wash 3 times with 50ml pure water each time, collect the organic phase, and dry over anhydrous sodium sulfate. After filtration, the solvent was spin-dried to obtain 4.5 g of the main product of acetophenone intermediate, 4-amino-3 bromoacetophenone, with a yield of 71%; at the same time, 1.2 g of by-product 4-amino-3,5-dibromoacetophenone was obtained , the yield is 14%, and can be used in Example 2 as a raw material for the synthesis of brobuterol.
[0069] Synthe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com