Method for using propylene epoxide in homogeneous system with low propylene using amount
A propylene oxide and usage-amount technology, which is applied in the production of bulk chemicals and organic chemistry, can solve the problems of low conversion rate of hydrogen peroxide to propylene oxide, high consumption of catalyst and energy, and low selectivity of propylene oxide. Achieve the effect of prolonging the stable operation time, less propylene consumption and reducing catalyst deactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
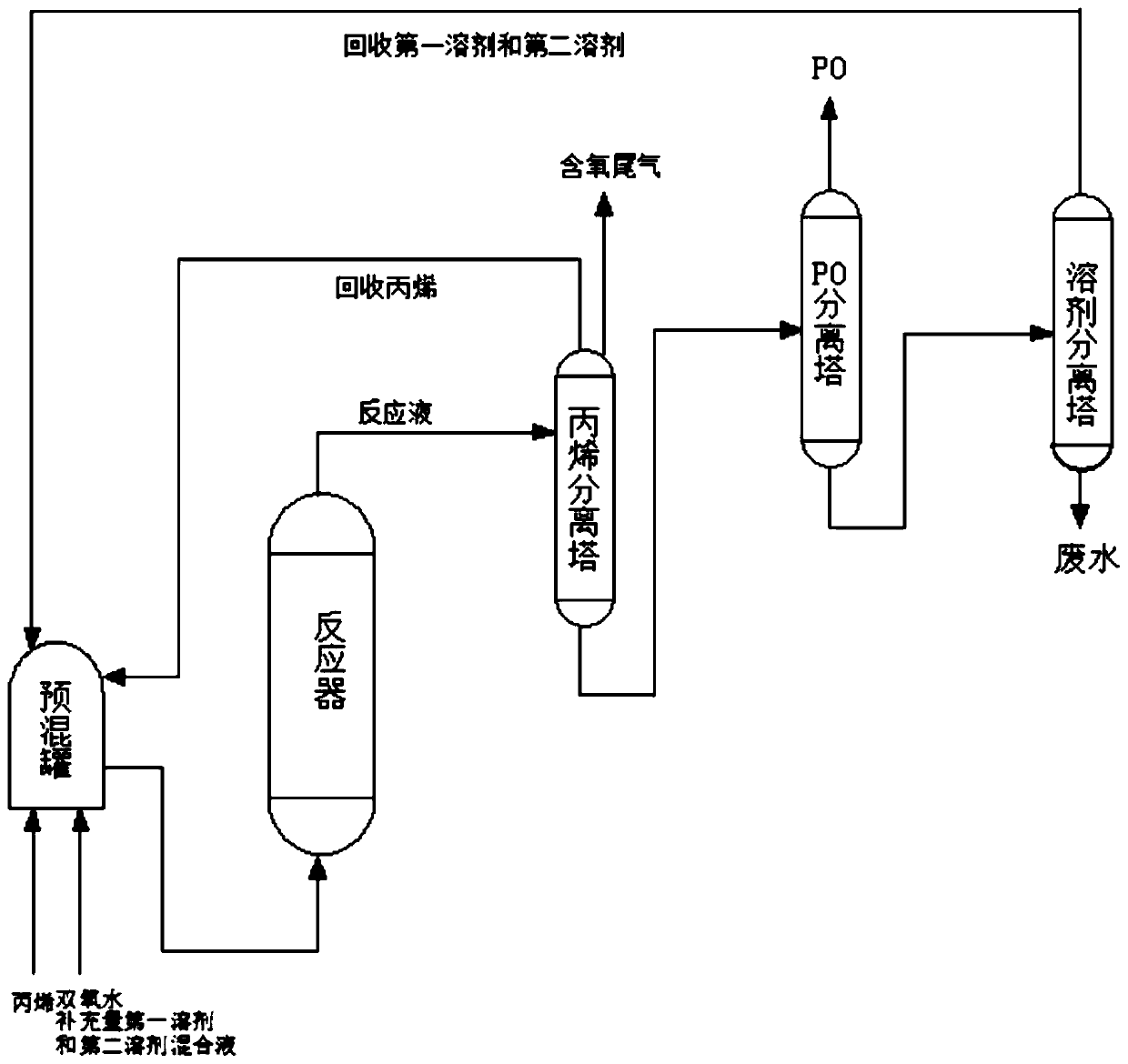
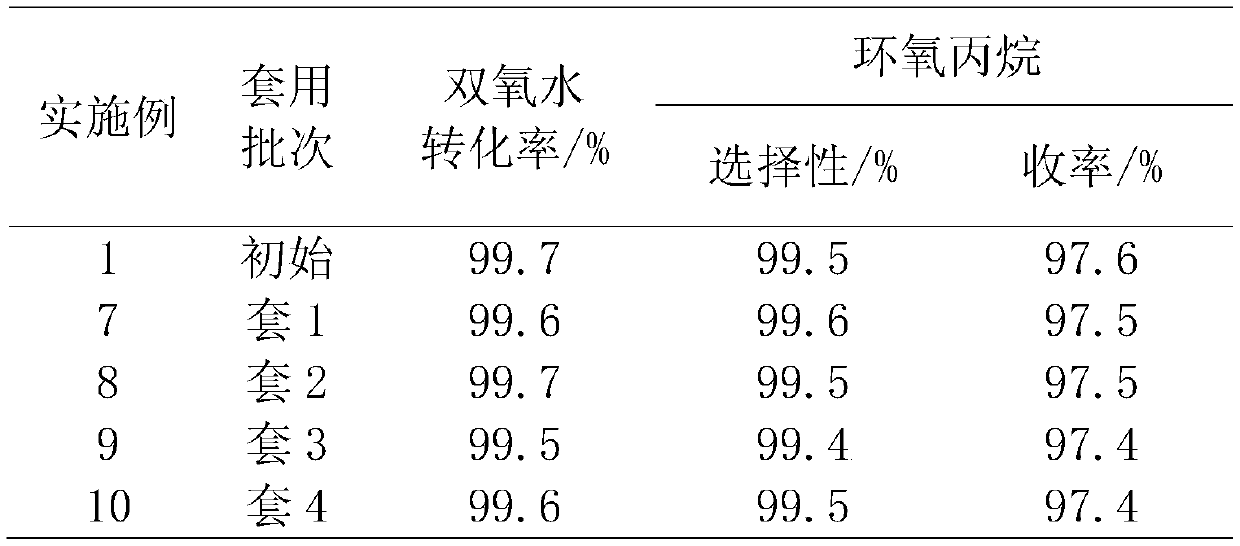
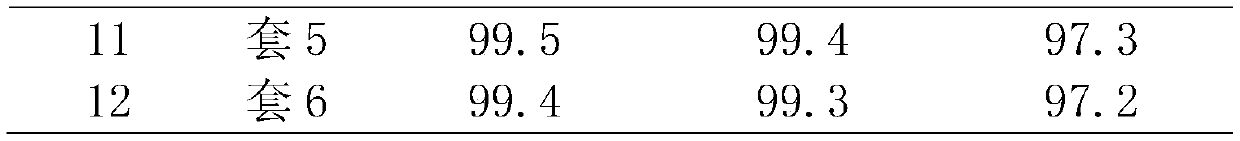
[0034] Mix 50% hydrogen peroxide, propylene, the first protic solvent methanol and the second aprotic solvent ether according to the molar ratio of hydrogen peroxide: propylene: methanol: ether equal to 1:2:4:2.5 in the premixing tank to form a homogeneous mixed liquid , and then at the total liquid mass space velocity 5h -1 , enter the titanium-silicon molecular sieve catalyst bed from the bottom of the reactor, control the temperature of the catalyst bed to 40°C, and the reaction pressure to 2.0MPa. After the reaction, the conversion rate of hydrogen peroxide is 99.7%, the selectivity of propylene oxide is 99.5%, and the yield of propylene oxide is 97.6%, tail oxygen content 0.05%, stable operation for 200h, unload part of the catalyst for thermogravimetric and BET analysis to determine the carbon content and pore volume of the catalyst, the results are shown in Table 2.
Embodiment 2
[0036] Mix 30% hydrogen peroxide, propylene, the first protic solvent ethanol and the second aprotic solvent methyl ethyl ketone according to the molar ratio of hydrogen peroxide: propylene: ethanol: methyl ethyl ketone equal to 1:2.5:6.6:1 in the premixing tank to form a homogeneous mixed liquid , and then at the total liquid mass space velocity 3h -1 , enter the titanium-silicon molecular sieve catalyst bed from the bottom of the reactor, control the temperature of the catalyst bed at 25°C, and the reaction pressure at 3.0MPa. After the reaction, the conversion rate of hydrogen peroxide is 99.1%, the selectivity of propylene oxide is 98.7%, and the yield of propylene oxide is 96.5%, tail oxygen content 0.06%, stable operation for 200h, unload part of the catalyst for thermogravimetric and BET analysis to determine the carbon content and pore volume of the catalyst, the results are shown in Table 2.
Embodiment 3
[0038] Put 60% hydrogen peroxide, propylene, the first protic solvent isopropanol and the second aprotic solvent cyclohexane according to the molar ratio of hydrogen peroxide: propylene: isopropanol: cyclohexane equal to 1:1.2:2.8:5 in the premix tank Mix in medium to form a homogeneous mixed liquid, and then use the total liquid mass space velocity for 1h -1 , enter the titanium-silicon molecular sieve catalyst bed from the bottom of the reactor, control the temperature of the catalyst bed to 35°C, and the reaction pressure to 0.5MPa. After the reaction, the conversion rate of hydrogen peroxide is 99.8%, the selectivity of propylene oxide is 98.5%, and the yield of propylene oxide is 95.4%, tail oxygen content 0.10%, stable operation for 200h, unload part of the catalyst for thermogravimetric and BET analysis to determine the carbon content and pore volume of the catalyst, the results are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com