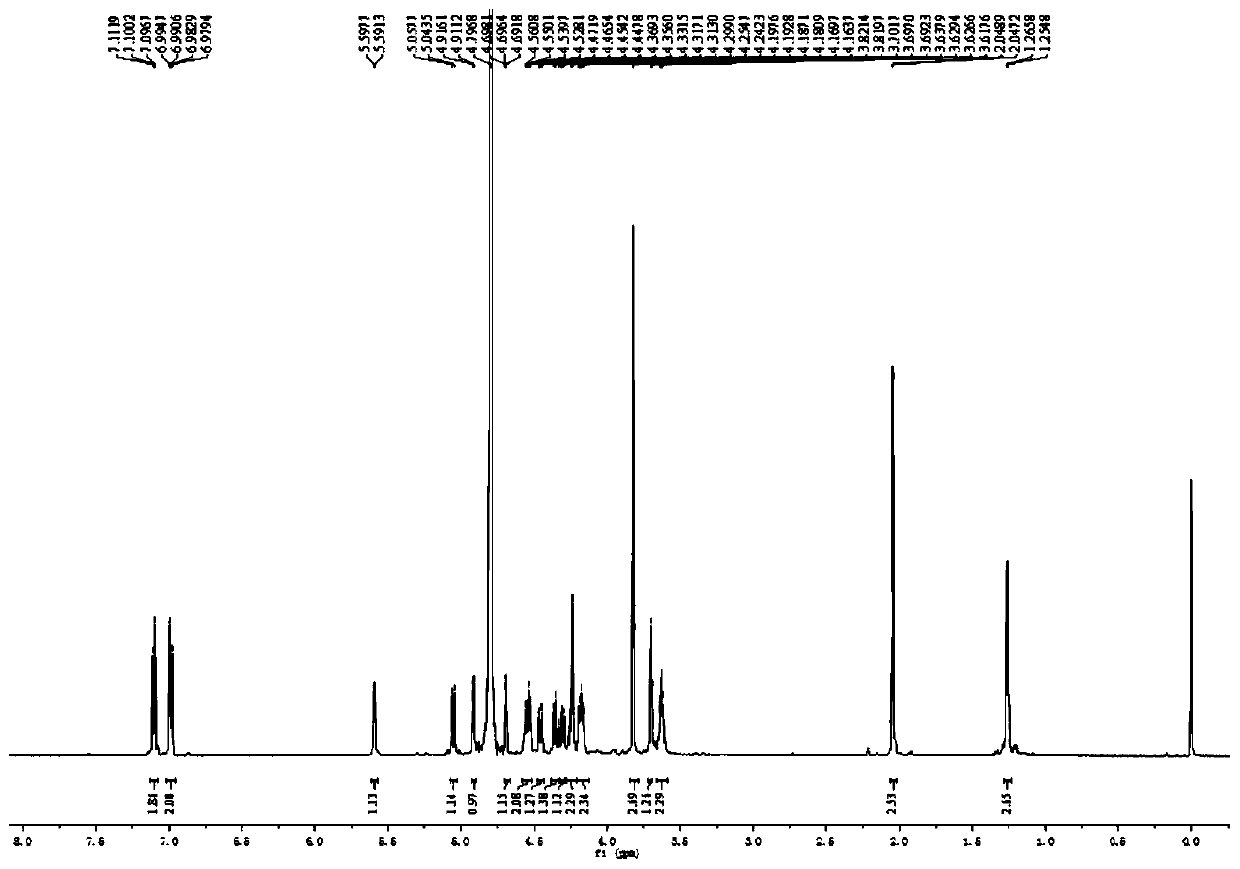
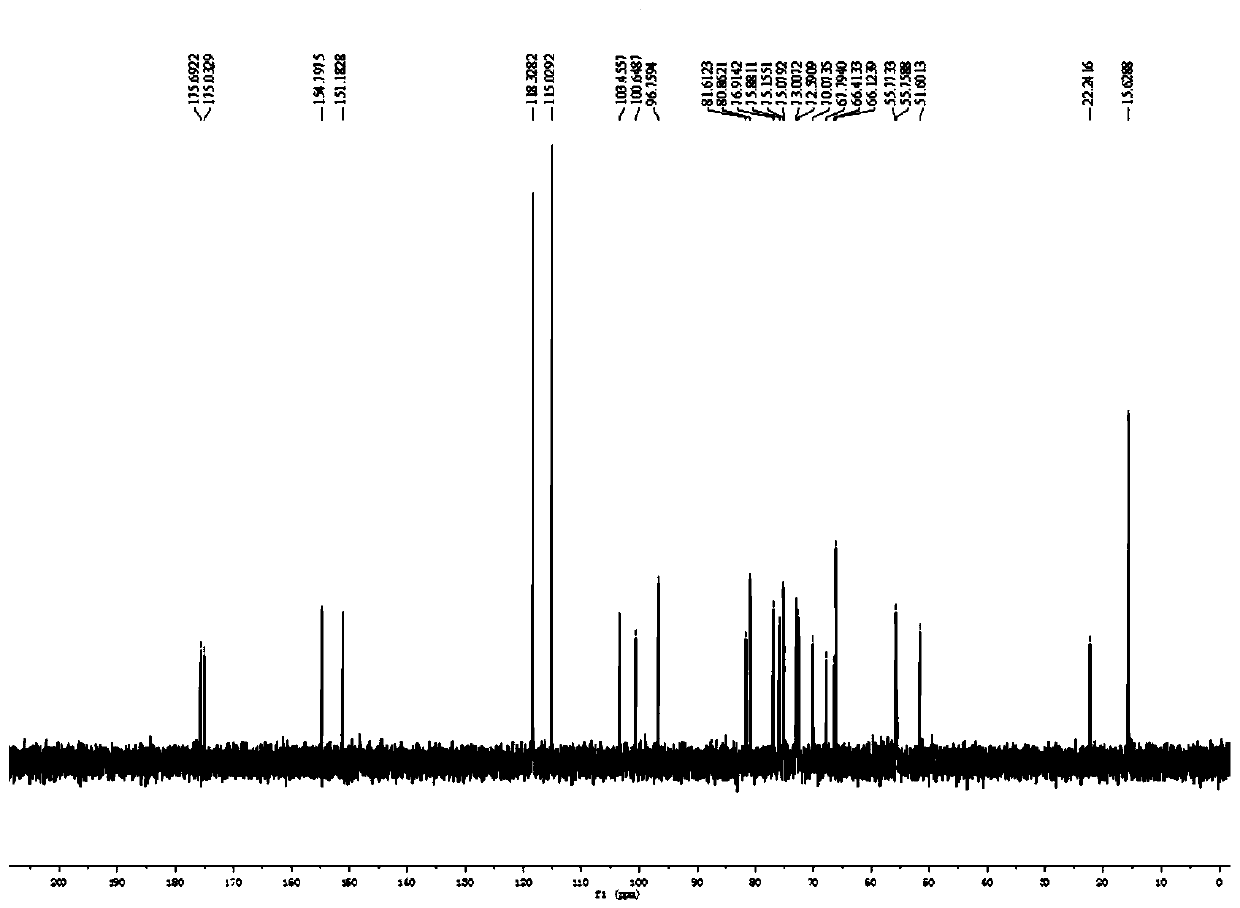
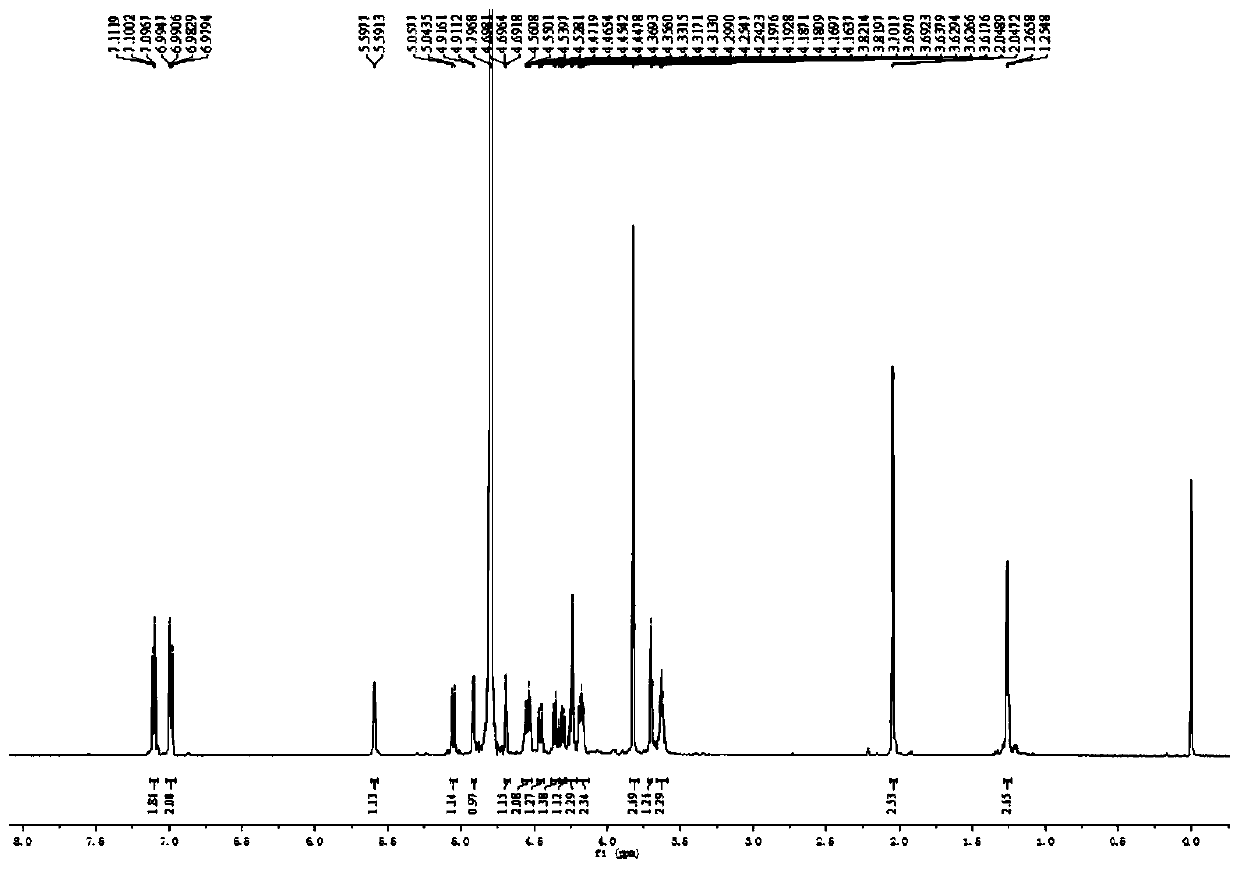
Preparation method, intermediate and application of fucosylated chondroitin sulfate trisaccharide
A chondroitin sulfate and fucosification technology, which is applied in the preparation of sugar derivatives, chemical instruments and methods, esterification saccharides, etc., can solve the problems of low yield, cumbersome fucose side chain connection steps, and no chemical Synthesize fucosylated chondroitin sulfate trisaccharide repeating units, etc., to achieve the effect of high-efficiency technical route and strong anti-inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Synthesis of glucuronic acid donor 7:
[0060] Using commercially produced β-pentaylglucose 15 as a raw material, first add glucosinolate to the 1-position, and then remove the acetyl group in sodium methoxide, and "one-pot method" protect the 4 and 6 positions with benzaldehyde acetal to obtain 14, Then selectively protect the 3-position with a p-methoxybenzyl group under low temperature conditions, and then protect the 2-position with a benzoyl group, and then remove the acetal under the condition of 80% acetic acid, and the 2,2,6,6- Under the conditions of tetramethylpiperidine oxide and iodobenzene diacetate, the 6-position was selectively oxidized to an acid and protected with benzyl to obtain 10, and finally the 4-position was protected with levulinyl to obtain the glucuronic acid donor 7. (Ac=acetyl, Ph=phenyl, Bn=benzyl, Bz=benzoyl, Lev=levulinyl, PMB=p-methoxybenzyl, Tol=p-tolyl)
[0061]
[0062] Synthesis of Compound 14:
[0063] Dissolve β-pentaacetylgl...
Embodiment 2
[0082] Synthesis of the galactosamine receptor:
[0083] Using D-galactosamine as raw material, use 1H-imidazole-1-sulfonyl azide hydrochloride to convert the 2-position to azide, and then obtain the acetylated product 17 under the conditions of pyridine and acetic acid. In trifluoromethanesulfonic acid Under the conditions of p-methoxyphenol and p-methoxyphenol, the terminal group of p-methoxyphenol product 16 was obtained, followed by deacetylation with sodium methoxide, and the 4 and 6 positions were protected with benzaldehyde acetal, and the acceptor 8 was obtained in one pot. (MP is p-methoxyphenyl)
[0084]
[0085] Synthesis of compound 17:
[0086] Dissolve galactosamine hydrochloride (5g, 23.25mmol) in 100mL of methanol, then add potassium carbonate (8g, 58.13mmol), anhydrous copper sulfate (42mg, 0.23mmol), 1H-imidazole-1-sulfonyl azide Nitrogen hydrochloride (6g, 34.88mmol), the reaction solution was stirred at room temperature for 3 hours, TLC monitored the r...
Embodiment 3
[0095] Synthesis of Fucose Donor 9:
[0096] The fucose donor, 9, is accomplished by the following steps. Using L-fucose as raw material, under the conditions of pyridine and acetic anhydride, the peracetylated product was obtained, and then directly reacted with p-cresylthiophenol to obtain the βthiophenol product 20, and then deacetylated in sodium methoxide, and dibutyl Under the condition of tin oxide, the selective benzyl at the 3-position gave 19, and the remaining hydroxyl was protected with allyloxycarbonyl to give the donor 9. (Alloc=allyloxycarbonyl)
[0097]
[0098] Synthesis of compound 20:
[0099] L-fucose (1 g, 6.10 mmol) was dissolved in 10 mL of pyridine and 5 mL of acetic anhydride, and reacted at room temperature for 8 h. TLC showed that the reaction was complete, and the solvent was spin-dried to obtain a yellow liquid (2.02 g, 100%). Then the yellow liquid and p-cresol (1.5g, 12.16mmol) were dissolved in 30mL of dichloromethane, and boron trifluorid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com