Unstratified metal sulfide nanosheet and preparation method thereof
A technology of metal sulfides and nanosheets, applied in the preparation of sulfides/polysulfides, chemical instruments and methods, lead sulfide, etc., can solve problems such as difficult kinetic factor regulation, difficult formation of synthesis methods, unfavorable nanocrystals, etc. , to achieve good application prospects, easy preparation, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A preparation method of non-layered lead sulfide nanosheets, comprising the steps of:
[0054] 1) Weigh 0.2 mmol of lead acetate and 0.4 mmol of n-dodecanethiol into a quartz flask, add 5 mL of oleylamine and 5 mL of oleic acid, and stir magnetically until a cloudy and uniform yellow solution is obtained.
[0055] 2) The above solution was heated in an oil bath at 75° C. for 30 min to obtain a clear and transparent yellow solution.
[0056] 3) Introduce argon gas into the clear and transparent yellow solution, and while heating at 75°C in an oil bath, select a 500W high-pressure mercury lamp as the light source for 4 hours of light, and then cool down to room temperature naturally to obtain a black turbid solution.
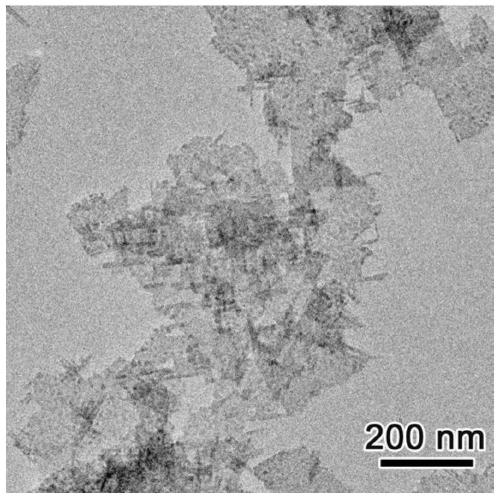
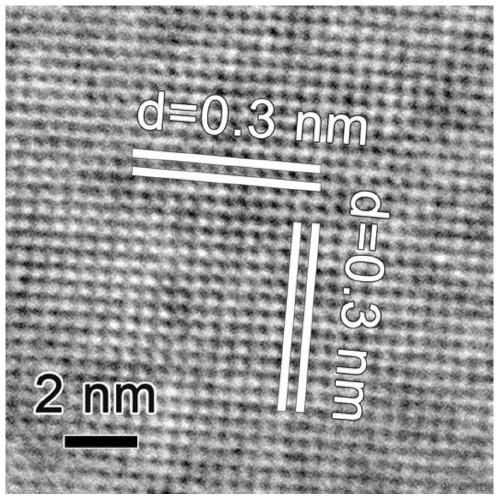
[0057] 4) Add toluene to the above black turbid solution and mix well, centrifuge at 6000 rpm for 5 minutes, repeat this process 5 times, and dry at 60-80°C for 12-24h to obtain non-layered PbS nanosheets .
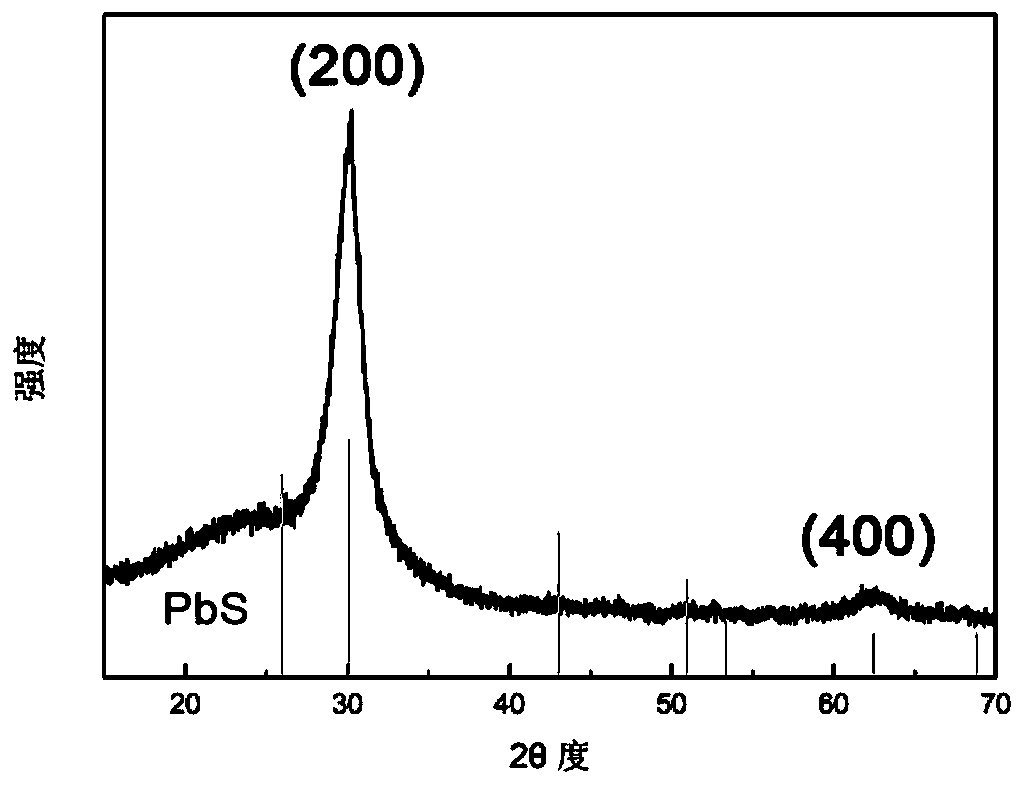
[0058] figure 1 The middle curve is the XRD s...
Embodiment 2
[0063] Repeat Example 1, the difference is only that the metal salt is changed from lead acetate to cadmium chloride, oleic acid is changed to octadecene, and the heating temperature is changed from 75°C to 120°C.
[0064] Figure 4 The middle curve is the XRD spectrum of the CdS nanosheets prepared in Example 1.
[0065] Figure 5 The transmission electron microscope image of the CdS nanosheets prepared in Example 2.
[0066] Figure 6 High-resolution transmission electron microscope image of the CdS nanosheets prepared in Example 2.
[0067] Depend on Figure 4 It can be seen that only the peak of CdS crystal appears in the XRD spectrum of the synthesized CdS nanosheets, indicating that the synthesized product is a pure phase of CdS. Depend on Figure 5 It can be seen that only flaky products are observed in the TEM images of the synthesized CdS nanosheets, which also shows that the synthesized CdS is nanosheets. Depend on Figure 6 It can be seen that the synthesize...
Embodiment 3-4
[0069] Repeat Example 1, its difference is only to change mercaptan into octyl mercaptan or n-octyl mercaptan. The obtained PbS has a non-layered nanosheet structure.
[0070] Figure 7 , 8 Shown are the transmission electron micrographs of the PbS nanosheets synthesized in Examples 3 and 4. In the figure, the PbS products synthesized by using two kinds of alkyl chain thiols have only non-layered nanosheet structure, and no nanoparticles or other morphological products appear.
[0071] The experiment of the present invention proves that the length of the straight-chain alkylthiol under investigation has no obvious influence on the formation of sulfide nanosheets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com