Preparation of basic blue-3 based near-infrared fluorescent probe molecule for hypochlorous acid detection
A technology for detecting hypochlorous acid and fluorescent probes, which is applied in the fields of fluorescence/phosphorescence, luminescent materials, material excitation analysis, etc. It can solve the problems of low sensitivity, complex synthesis and slow response speed of hypochlorous acid detection probes, and achieve rapid The effect of hypochlorous acid response, high sensitivity and short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
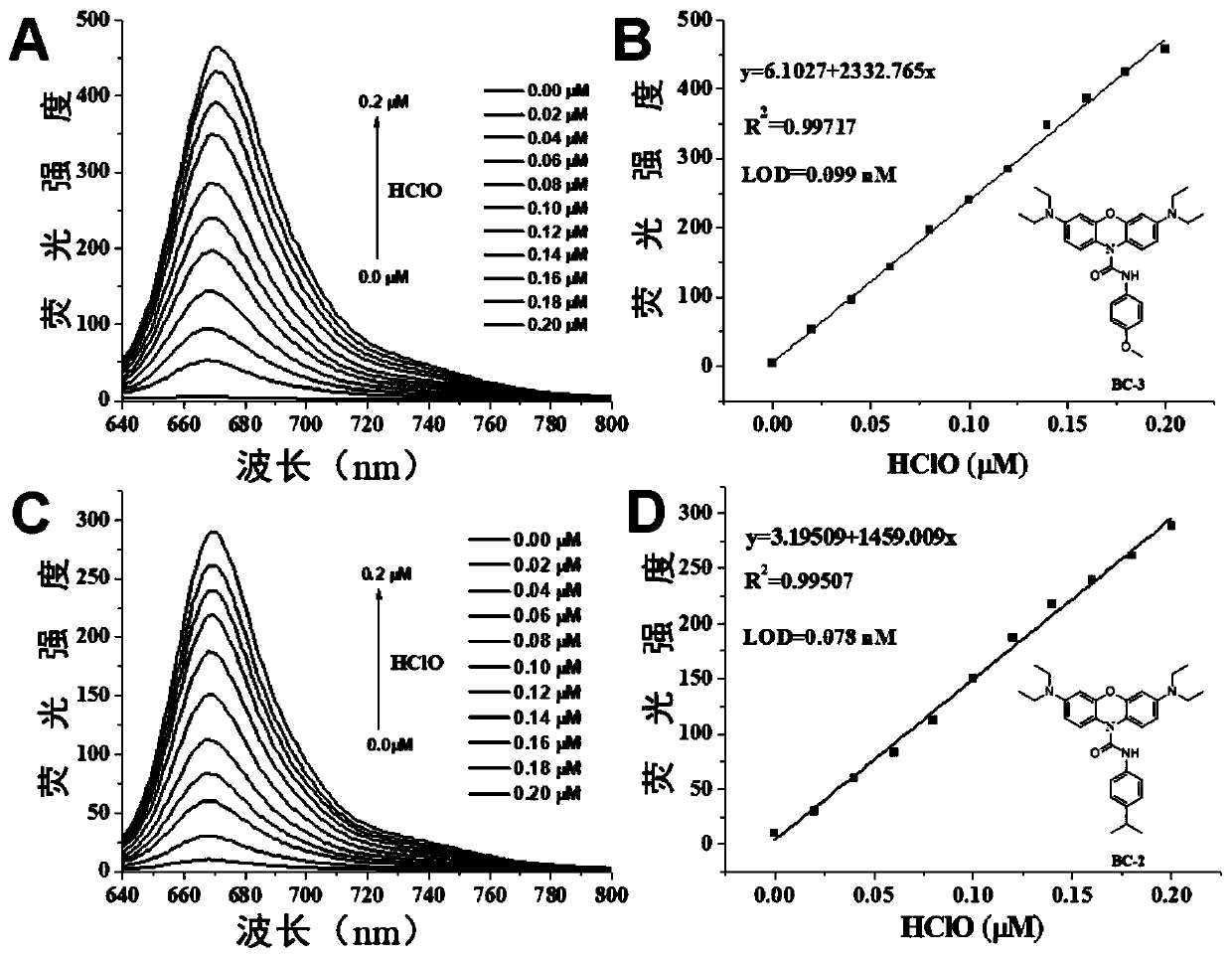
[0044] The near-infrared fluorescent probe molecule provided in this embodiment is a near-infrared fluorescent probe molecule for detecting hypochlorous acid based on basic blue-3, and its structural formula is:
[0045]
[0046] In the above structural formula: X is different electron-withdrawing groups and electron-donating groups, hydrogen atoms, alkyl or cycloalkyl, substituted alkyl or cycloalkyl.
[0047] The near-infrared fluorescent probe molecule can use an alkyl or cycloalkyl group with less than 6 carbons.
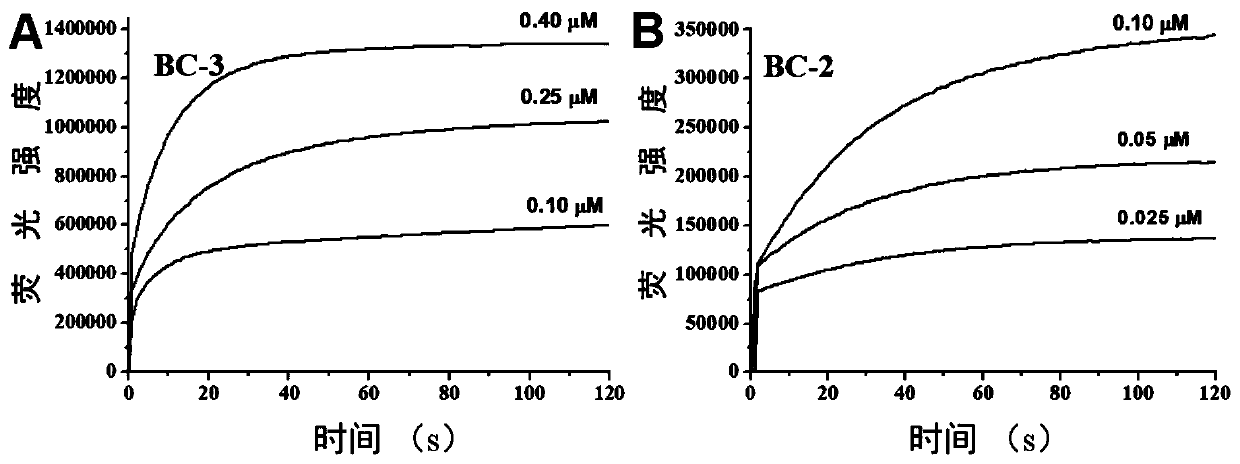
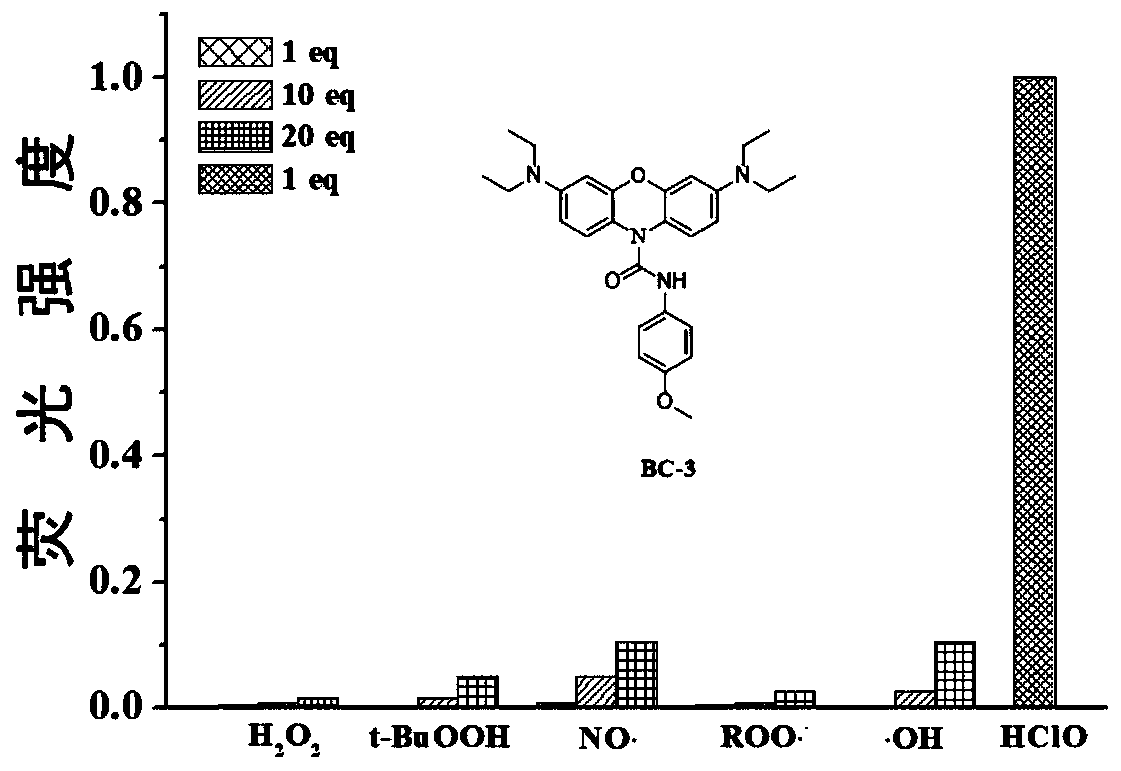
[0048] The near-infrared fluorescent probe molecule is used for preparing rapid response and non-invasive specific detection hypochlorous acid probe, especially for specific detection of hypochlorous acid.
[0049] In the following examples, two compounds BC-2 and BC-3 are prepared and described in detail by taking them as examples.
[0050]
[0051] In the above figure, BB-3 is the basic blue-3 molecule, BC is the product of BB-3 acid chloride, 3 is p-cy...
Embodiment 2
[0055] Embodiment 2 (BC-2):
[0056] The preparation of compound BC-2: add compound BC (50mg, 0.13mmol) in the schlenk tube of 100mL, Na 2 CO 3 (55.4mg, 0.52mmol), p-cymeniline (70.3mg, 0.52mmol), and the solvent dichloromethane (5.0mL) was added. The reaction solution was reacted at 40° C. for 8 h until the reaction was complete. After the reaction, the reaction liquid was cooled to room temperature, concentrated under reduced pressure, separated and purified by column chromatography to obtain compound BC-2 (light blue solid, 64.8%; ethyl acetate:petroleum ether=1:10).
[0057] 1 H NMR (400MHz, CDCl 3 )δ=7.43–7.37(m,2H),7.35(d,J=8.5Hz,2H),7.23(s,1H),7.15(d,J=8.4Hz,2H),6.44(s,4H), 3.36(q, J=7.0Hz, 8H), 2.87(dt, J=13.8, 6.9Hz, 1H), 1.26–1.16(m, 18H).
[0058] 13 C NMR: (100MHz, CDCl 3 )δ=153.25, 152.50, 146.81, 143.86, 136.22, 126.77, 125.04, 119.73, 117.60, 106.74, 100.07, 44.65, 33.54, 24.13, 12.58.
[0059] HR-MS(ESI,m / z):calcd for C 30 h 38 N 4 o 2 [M+H] + ,48...
Embodiment 3
[0060] Embodiment 3 (BC-3):
[0061] The preparation of compound BC-3: add compound BC (50mg, 0.13mmol) in the schlenk tube of 100mL, Na 2 CO 3 (55.4mg, 0.52mmol), p-methoxyaniline (64.0mg, 0.52mmol), added solvent dichloromethane (5.0mL). The reaction solution was reacted at 40° C. for 8 h until the reaction was complete. After the reaction, the reaction solution was cooled to room temperature, concentrated under reduced pressure, separated and purified by column chromatography to obtain compound BC-3 (off-white solid, 65.3%; ethyl acetate:petroleum ether=1:5).
[0062] 1 H NMR (400MHz, CDCl 3 )δ=7.40–7.35(m,2H),7.32(d,J=9.0Hz,2H),7.15(s,1H),6.82(d,J=9.0Hz,2H),6.43(d,J=2.7 Hz,4H),3.77(s,3H),3.34(q,J=7.0Hz,8H),1.16(t,J=7.0Hz,12H).
[0063] 13 C NMR (100MHz, CDCl 3 )δ=155.82, 153.60, 152.49, 146.80, 131.59, 125.06, 121.69, 117.58, 114.04, 106.72, 100.04, 55.50, 44.64, 12.57.
[0064] HR-MS(ESI,m / z):calcd for C 28 h 34 N 4 o 3 [M+H] + ,475.2664,found 475.2735.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com