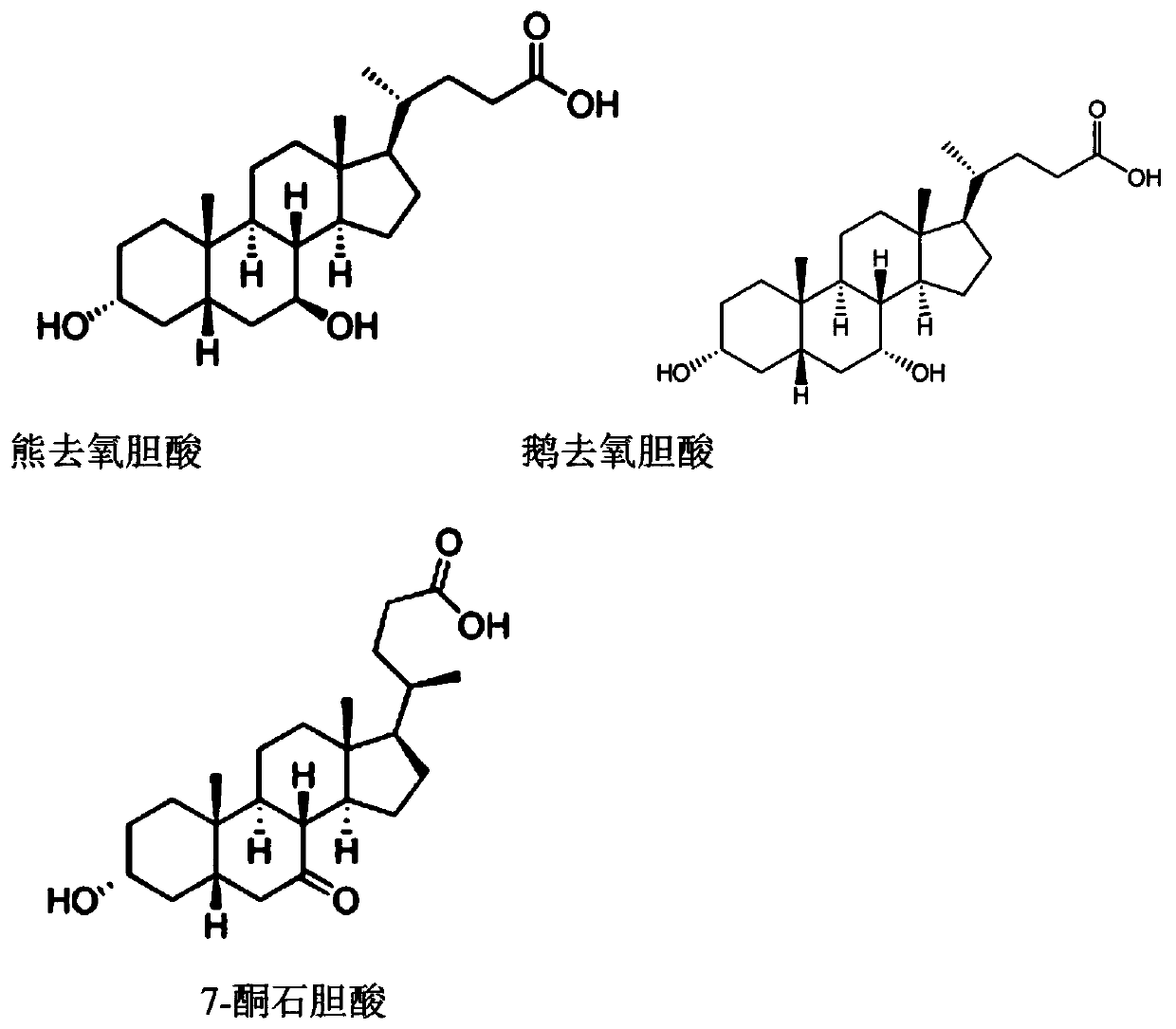
Production process of ursodeoxycholic acid
A technology for the production of ursodeoxycholic acid, which is applied in the chemical field, can solve the problems of low product quality, unsafe production process, and large consumption of organic solvents, and achieve the effects of high automation, pollution avoidance, and purity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
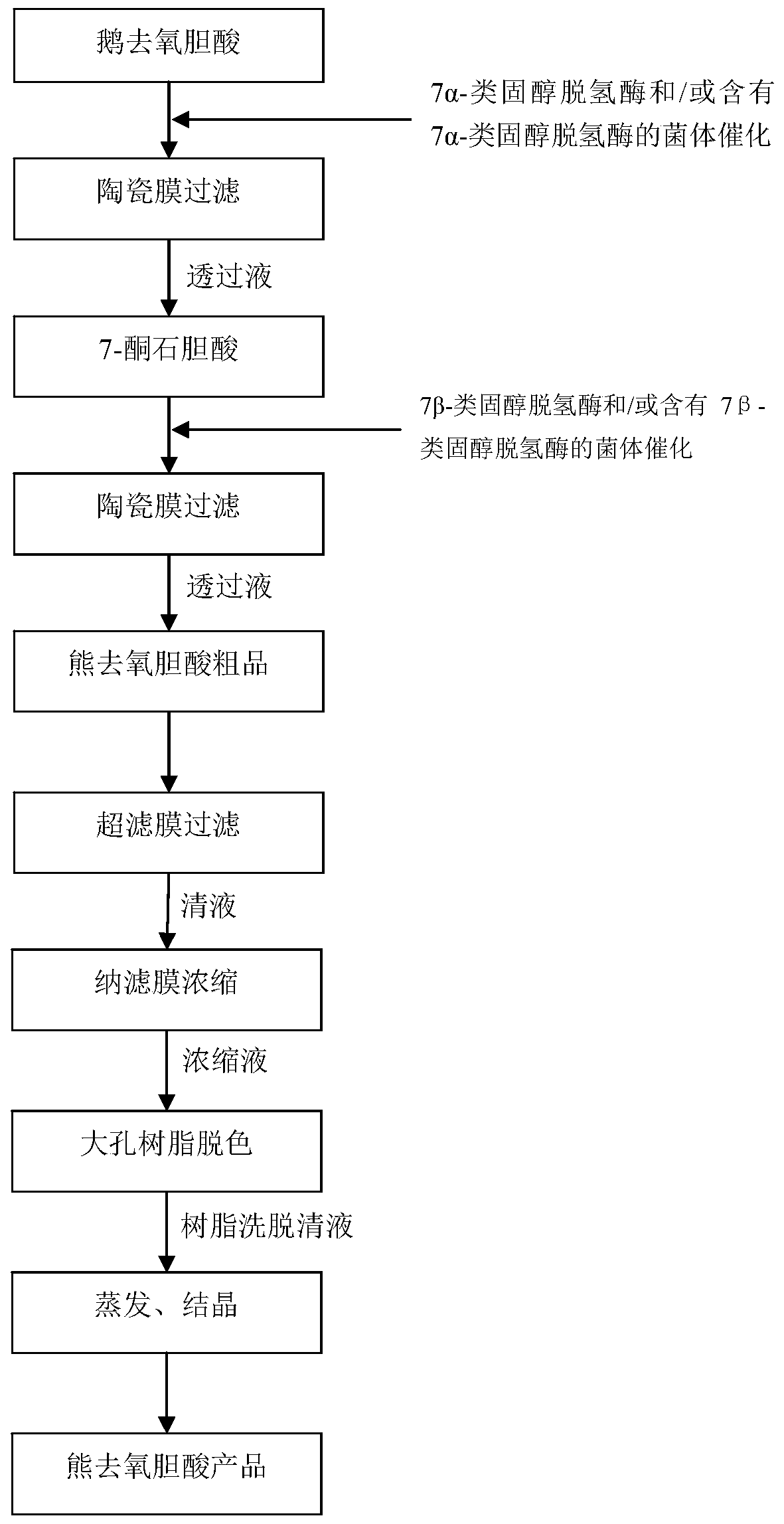
[0044] as per figure 1 The flow chart for the preparation of ursodeoxycholic acid is shown:
[0045] (1) catalyzing chenodeoxycholic acid through 7α-steroid dehydrogenase, the addition amount is 0.001% of the volume of chenodeoxycholic acid, and the catalysis temperature is 20°C to obtain the catalytic product A;
[0046] (2) The catalytic product A obtained in step (1) is filtered through a microfiltration membrane (the microfiltration membrane is a ceramic membrane, the filtration accuracy is 5nm, the filtration temperature is 20°C, and the filtration pressure is 0.8MPa), to obtain bile acid permeate;
[0047] (3) Add 7β-steroid dehydrogenase to the permeate containing 7-ketolithocholic acid obtained in step (2), in an amount of 0.001% of the volume of the permeate containing 7-ketolithocholic acid, The catalytic temperature is 20°C, and the catalytic reaction is carried out to obtain the catalytic product B;
[0048] (4) The catalytic product B obtained in step (3) is fi...
Embodiment 2
[0055] as per figure 1 The flow chart for the preparation of ursodeoxycholic acid is shown:
[0056] (1) catalyzing chenodeoxycholic acid through bacteria containing 7α-steroid dehydrogenase, the addition amount is 2% of the volume of chenodeoxycholic acid, and the catalysis temperature is 60°C to obtain the catalytic product A;
[0057] (2) The catalytic product A obtained in step (1) is filtered through a microfiltration membrane (the filtration precision is 500 nm, the filtration temperature is 80° C., and the filtration pressure is 0.1 MPa), to obtain a permeate containing 7-ketolithocholic acid;
[0058] (3) In the permeate solution containing 7-ketolithocholic acid obtained in step (2), add the bacterium containing 7β-steroid dehydrogenase, and the amount added is the volume of the permeate solution containing 7-ketolithocholic acid 2%, the catalytic temperature is 60°C, and the catalytic reaction is carried out to obtain the catalytic product B;
[0059] (4) The catal...
Embodiment 3
[0066] as per figure 1 The flow chart for the preparation of ursodeoxycholic acid is shown:
[0067] (1) catalyzing chenodeoxycholic acid through 7α-steroid dehydrogenase, the addition amount is 0.05% of the volume of chenodeoxycholic acid, and the catalysis temperature is 20°C to obtain the catalytic product A;
[0068] (2) The catalytic product A obtained in step (1) is filtered through a microfiltration membrane (the microfiltration membrane is a ceramic membrane, the filtration accuracy is 200nm, the filtration temperature is 20°C, and the filtration pressure is 0.2MPa), to obtain bile acid permeate;
[0069] (3) Add 7β-steroid dehydrogenase to the permeated liquid containing 7-ketolithocholic acid obtained in step (2), in an amount of 0.05% of the volume of the permeated liquid containing 7-ketolithocholic acid, The catalytic temperature is 20°C, and the catalytic reaction is carried out to obtain the catalytic product B;
[0070] (4) The catalytic product B obtained i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com