Application of N6-(2-hydroxyethyl) adenosine in medicine for treating hypertension
A kind of hydroxyethyl, high blood pressure technology, applied in the application field of N6-adenosine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1N6-(2-hydroxyethyl) adenosine (HEA) etc. obtain
[0032]N6-(2-Hydroxyethyl)adenosine (HEA) is an adenosine derivative extracted from Cordyceps mycelium. It is a calcium antagonist (calciumAntagonists, CCB) of biological origin and can also be synthesized artificially.
[0033] The molecular structural formula of N6-(2-hydroxyethyl)adenosine (HEA) is as follows:
[0034]
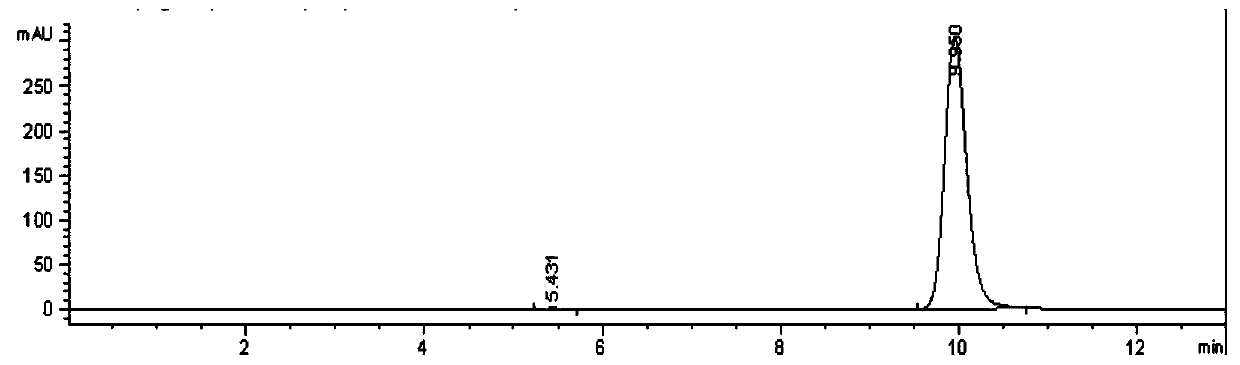
[0035] The laboratory planted artificial Cordyceps mycelium and used wheat as the medium to greatly reduce the content of heavy metals. The cicada was dried and crushed into 200 mesh particles. The extraction and separation of HEA: using 15%-75% ethanol as the solvent , the ratio of solvent to mycelia is 10mL / g, and the ethanol extract is obtained at a temperature of 50°C–100°C; the ethanol extract is intercepted by an ultrafiltration membrane to obtain a concentrate; the membrane concentrate is purified by AB-8 macroporous resin , after eluting the impurities, collect 15% and 30% ethan...
Embodiment 2
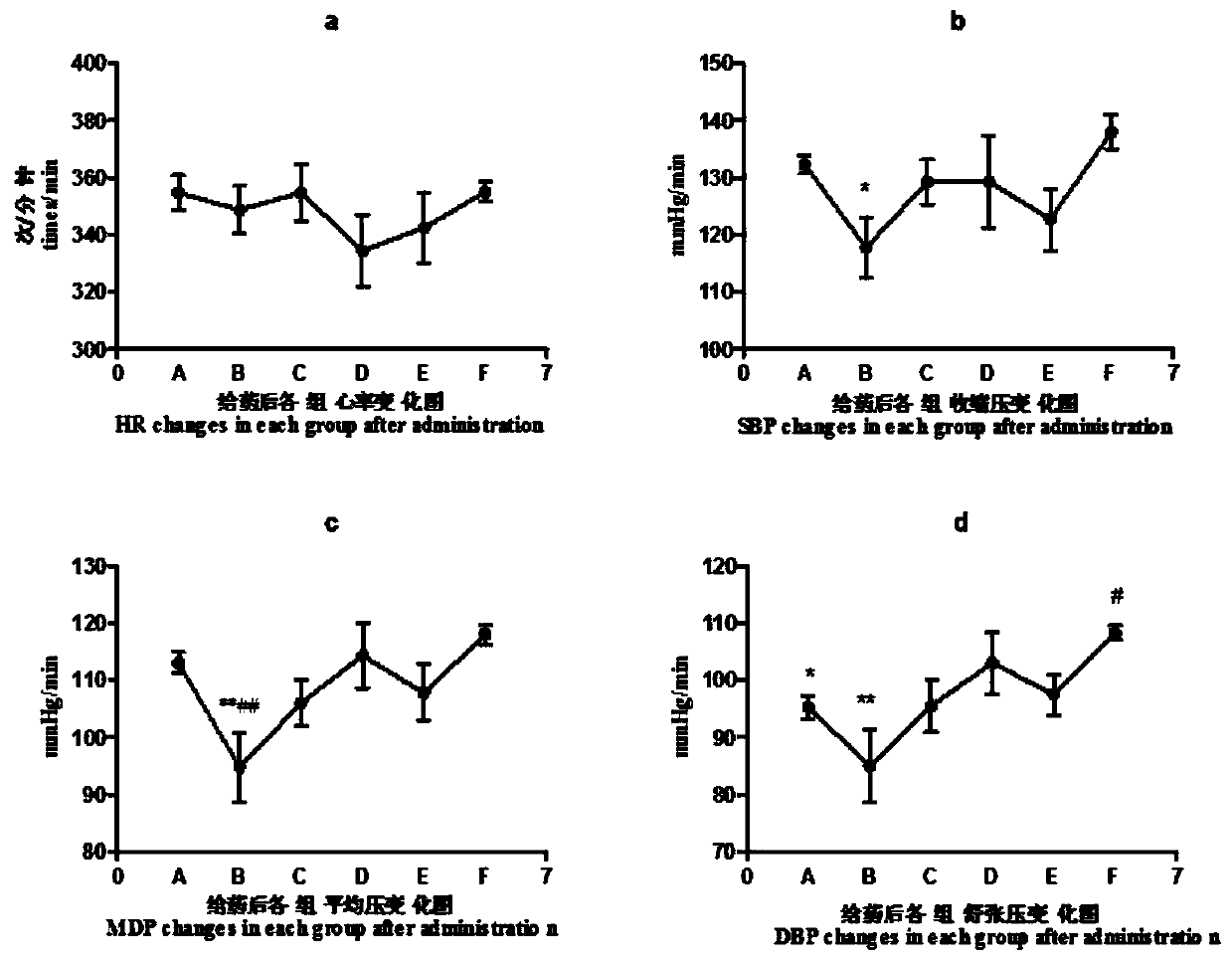
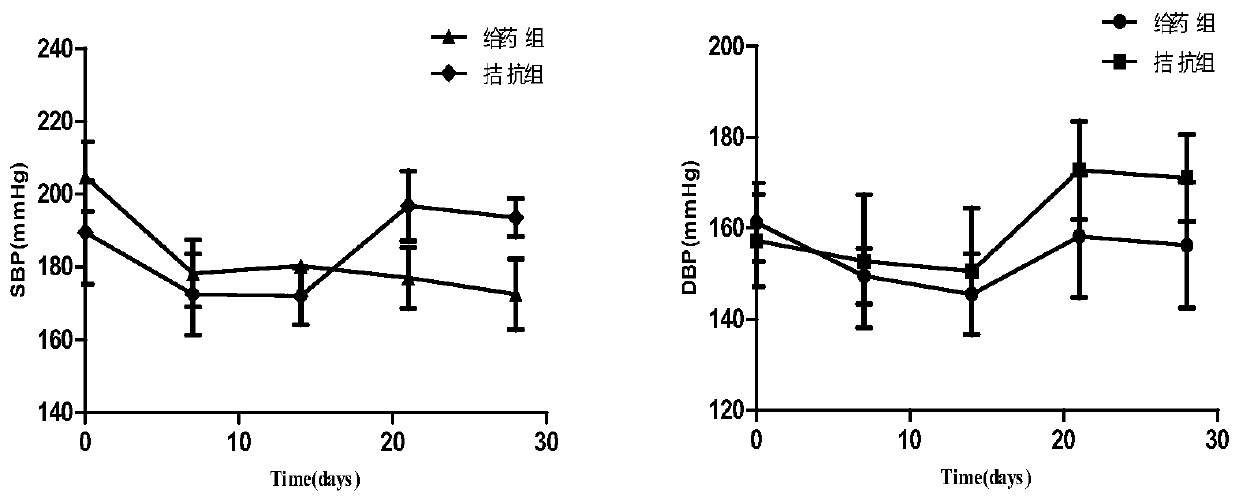
[0039] Example 2: The antihypertensive effect of N6-(2-hydroxyethyl)adenosine (HEA) on SD rats in hypertensive model.
[0040] Materials and Reagents:
[0041] Experimental animals: 20 male SD rats, 9 weeks old, weighing 200 ± 10g, purchased from the Animal Experiment Center of Wenzhou Medical University, animal quality certificate number: SCXK (Zhejiang) 2017-149, animals were raised in an independent animal room, room temperature 20-25°C, alternating light and dark light for 12 hours, relative humidity 50%, free access to food, water and feed, and adaptive feeding for one week.
[0042] Drugs and instruments: SoftronBP 2010 non-invasive blood pressure measuring instrument was purchased from Beijing Ruanlong Biotechnology Co., Ltd.; selective A1R antagonist DPCPX was purchased from Sigma Company; NO synthase inhibitor NG-nitro-L-arginine methyl ester (NG -Nitro-L-arginine methyl ester hydrochloride, L-NAME) was purchased from Sigma Company, and the required concentration was...
Embodiment 4
[0073] Example 4 Binding site of HEA-HSA system
[0074] According to the Scatchard equation, the binding constant (KA) and the number of binding sites (n) during the binding process were determined (Gao et al. 2004).
[0075]
[0076] In the formula, F0 is the fluorescence intensity of HSA, F is the fluorescence intensity of HEA-HSA complex, and [Q] is the concentration of HEA.
[0077] Measurement of fluorescence spectrum: Measure the fluorescence emission spectrum on a Thermo Scientific Lumina type fluorescence spectrometer, the excitation wavelength is 280nm, and the scanning range of the emission wavelength is 285nm to 500nm. A 3.5 mL solution of 3.0 μmol / L HSA was titrated by adding HEA. The excitation and emission slit widths are 2.5nm, the scanning speed is 600nm / min, the response time is 1.0s, the integration time is 20ms, and the photomultiplier tube voltage is 500V. Samples were tested at 298K and 308K.
[0078] Such as Figure 6 As shown, c(HSA)=3.0μmol / L, c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com