Extraction method of alkyl resorcinol in wheat bran
A technology of alkyl resorcinol and wheat bran, which is applied in the field of separation and purification of natural functional substances, can solve the problems of high price, high risk, and difficult control, and achieve toxicity, environmental protection, low price, and high utilization rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Raw material pretreatment: remove gravel, silt, and diseased insect grains from the wheat harvested in the same year, wash and dry, and use a mill to grind the wheat bran to 80 mesh after obtaining the bran;
[0039] (2) Extraction: Ultrasonic extraction of wheat bran was performed with ethyl acetate as the extraction solvent, the extraction time was 30 min, and the extraction power was 300 W;
[0040] (3) Centrifugation: Centrifuge at 3500 r / min for 10 minutes to obtain the sample supernatant;
[0041] (4) Concentration: Collect the supernatant obtained by centrifugation and concentrate it using a rotary evaporator at a temperature of 45 °C to obtain a concentrated solution of the alkyl resorcinol ethyl acetate extract;
[0042](5) Separation and purification: using silica gel column chromatography, first place 100-200 mesh silica gel powder in a 120 °C oven for high temperature activation for 30 min, and then place it in a desiccator to cool to room temperature. ...
Embodiment 2
[0056] (1) Raw material pretreatment: remove gravel, silt, and diseased insect grains from the wheat harvested in the same year, wash and dry, and use a mill to grind the wheat bran to 80 mesh after obtaining the bran;
[0057] (2) Extraction: Ultrasonic extraction of wheat bran was performed with ethyl acetate as the extraction solvent, the extraction time was 30 min, and the extraction power was 300 W;
[0058] (3) Centrifugation: Centrifuge at 3500 r / min for 10 minutes to obtain the sample supernatant;
[0059] (4) Concentration: Collect the supernatant obtained by centrifugation and concentrate it using a rotary evaporator at a temperature of 45 °C to obtain a concentrated solution of the alkyl resorcinol ethyl acetate extract;
[0060] (5) Separation and purification: select a solid phase extraction column, first use n-pentane to flush the column to remove impurities and reduce the polarity of the solid phase extraction column, and then load the concentrated solution of t...
Embodiment 3
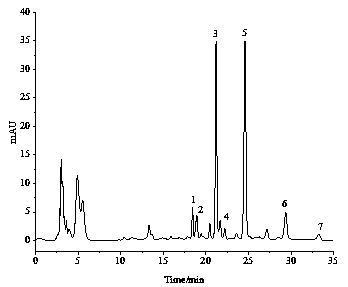
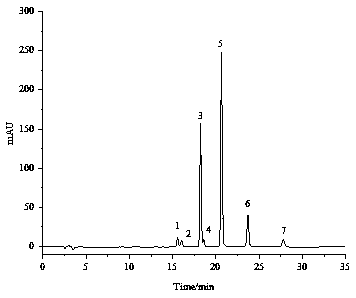
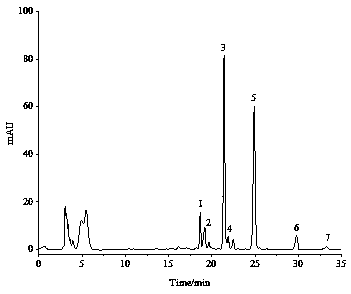
[0074] Comparing the separation and purification effects of various alkyl resorcinols, the mixture obtained in Example 1 has good separation and few impurities. The alkylresorcinol homologue mixture obtained in Example 1 was used to further prepare the alkylresorcinol monomer through the semi-preparative liquid phase.
[0075] The semi-preparative liquid chromatography conditions are:
[0076] (1) Mobile phase: A phase 0.1% formic acid aqueous solution; B phase 0.1% formic acid methanol solution, flow rate: 2 mL / min, column temperature: 30 ℃, injection volume: 200 µL;
[0077] (2) The mobile phase elution program is: 0min, A phase 15~25%, B phase 75~85%; 5min, A phase 15~10%, B phase 75~90%; 10min, A phase 5~10% , B phase 90~95%; 15min, A phase 0~5%, B phase 95~100%; 35min, A phase 15~25%, B phase 75~85%;
[0078] (3) Detector: DAD detector is used, and the detection wavelength is 280 nm.
[0079] Obtained 5 monomers of alkylresorcinol under the above-mentioned conditions, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com