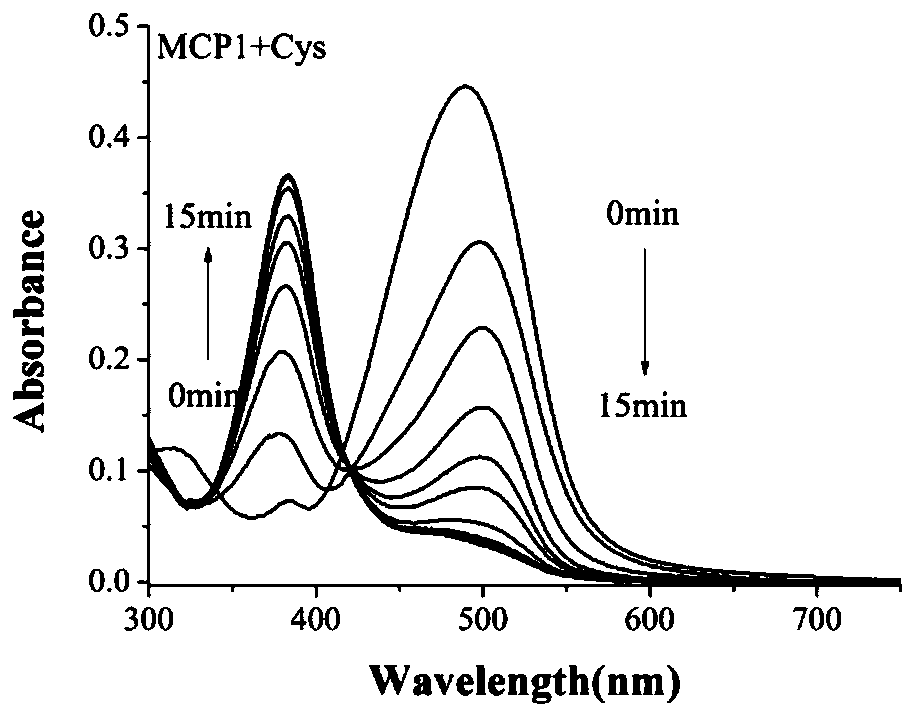
Fluorescent probe for specifically identifying hydrogen polysulphide and bio-thiol
A technology of polyhydrogen sulfide and fluorescent probes, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of not being able to distinguish at the same time, and achieve the effect of simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
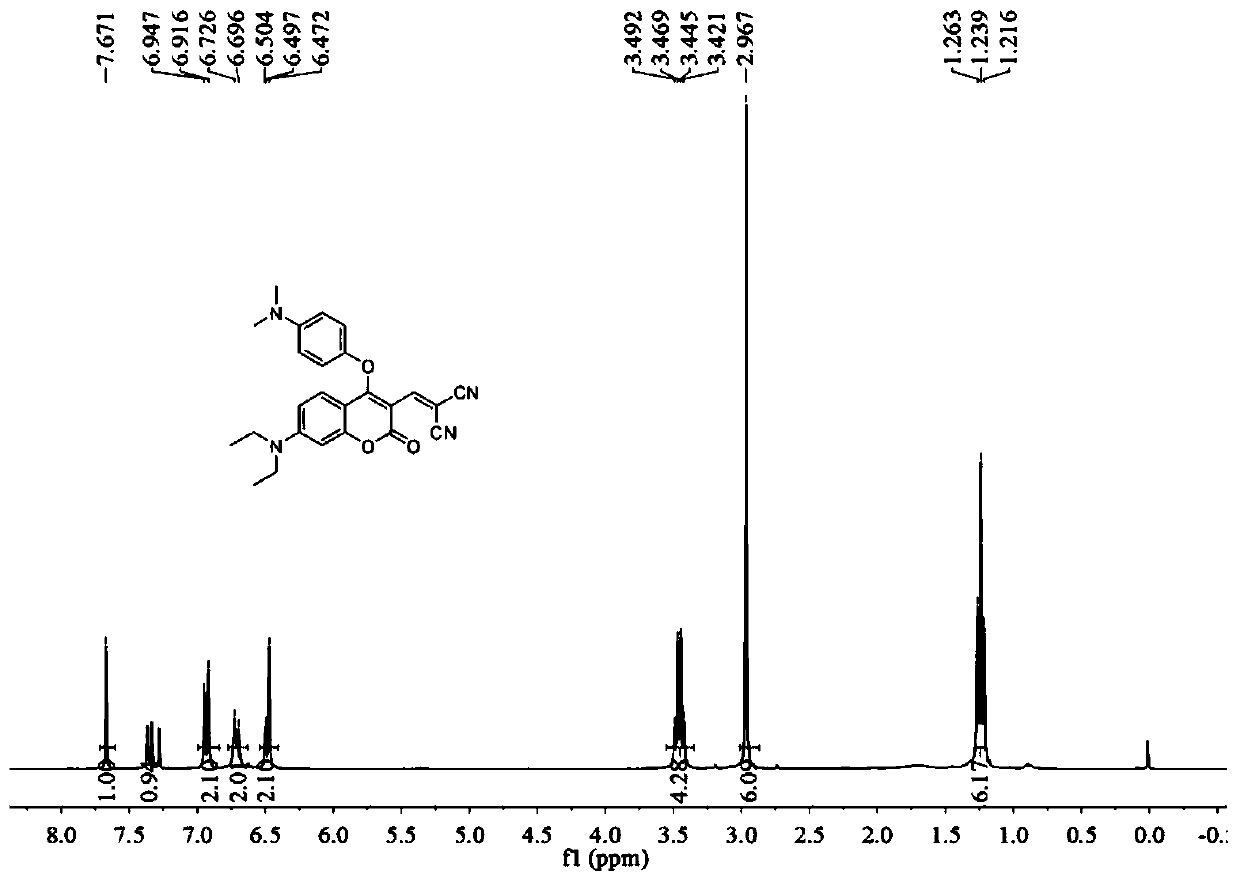
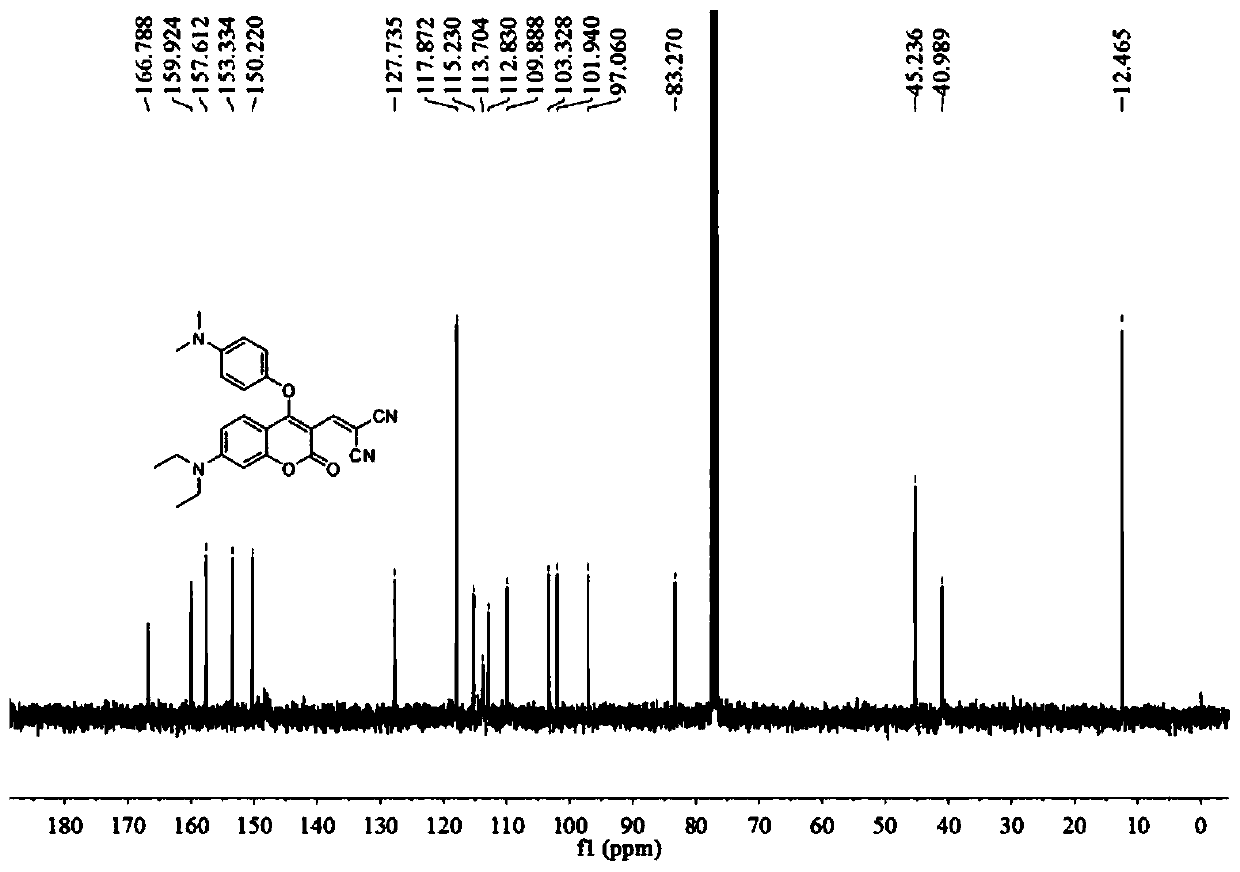
[0040] A fluorescent probe that specifically recognizes hydrogen polysulfide and biological thiols. The structural formula of the fluorescent probe is as follows:
[0041]
[0042] The preparation method of a fluorescent probe that specifically recognizes hydrogen polysulfide and biological thiols includes the following steps:
[0043] Step 1: Dissolve 3-aldehyde-4-chloro-7-diethylaminocoumarin (labeled 1,1mmol, 279mg) in 5mL of anhydrous acetonitrile, and then add 4-dimethylaminophenolate Acid salt (1mmol, 173mg), and finally triethylamine (NEt 3 ) (1mmol, 139μL) as a base, protected by argon, and reacted at 80°C under reflux for 4h. The solvent was spin-dried to obtain a mixture. The mixture was purified to obtain compound A. The mixture was a reddish-brown solid and was purified to obtain dark brown compound A, totaling 304 mg , The yield is 80%;
[0044] The main product in the mixture is compound A;
[0045] The reaction equation is:
[0046]
[0047] Step two, dissolve compound ...
Embodiment 2
[0052] A fluorescent probe that specifically recognizes hydrogen polysulfide and biological thiols. The structural formula of the fluorescent probe is as follows:
[0053]
[0054] The preparation method of a fluorescent probe that specifically recognizes hydrogen polysulfide and biological thiols includes the following steps:
[0055] Step 1. Dissolve 3-aldehyde-4-chloro-7-diethylaminocoumarin (labeled 1,1mmol, 279mg) in 10mL of anhydrous acetonitrile, and add 4-dimethylaminophenolate Acid salt (2mmol, 346mg), and finally triethylamine (NEt 3 ) (2mmol, 278μL) as a base, protected by argon, and reacted under reflux at 100°C for 4.5 hours. The solvent was spin-dried to obtain a mixture. The mixture was purified to obtain compound A. The mixture was a reddish brown solid and was purified to obtain dark brown compound A. Total 291.8mg, the yield was 76.8%;
[0056] The main product in the mixture is compound A;
[0057] The reaction equation is:
[0058]
[0059] Step two, dissolve compo...
Embodiment 3
[0063] A fluorescent probe that specifically recognizes hydrogen polysulfide and biological thiols. The structural formula of the fluorescent probe is as follows:
[0064]
[0065] The preparation method of a fluorescent probe that specifically recognizes hydrogen polysulfide and biological thiols includes the following steps:
[0066] Step 1: Dissolve 3-aldehyde-4-chloro-7-diethylaminocoumarin (labeled 1,1mmol, 279mg) in 15mL of anhydrous acetonitrile, and then add 4-dimethylaminophenolate Salt (3mmol, 519mg), and finally triethylamine (NEt 3 ) (3mmol, 417μL) as a base, protected by argon, and reacted at 90°C for 5 hours by rotating the solvent to dryness. The mixture was purified to obtain compound A, where the mixture was a reddish-brown solid and was purified to obtain dark brown compound A, totaling 297.2 mg, the yield is 78.2%;
[0067] The main product in the mixture is compound A;
[0068] The reaction equation is:
[0069]
[0070] Step 2: Dissolve compound A (297.2304mg, 0.7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com