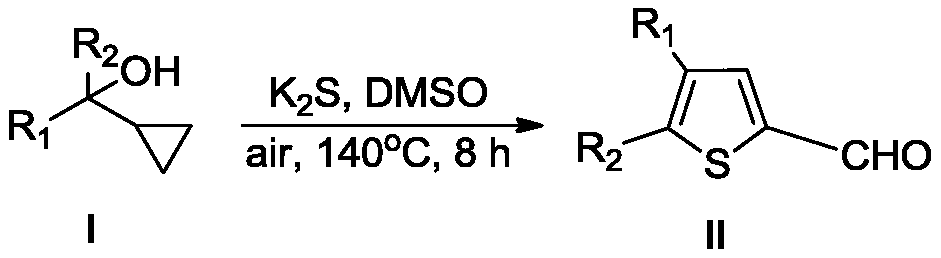
Novel method for preparing thiophene formaldehyde compounds
A technology of thiophene formaldehyde and compounds, which is applied in the field of preparation of multi-substituted thiophene formaldehyde compounds, can solve the problems of complex reaction steps, high industrial cost, harsh reaction conditions, etc., and achieve the effects of short reaction time, simple operation and simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation method of the present embodiment comprises the following steps:
[0038] Add compound Ia (0.06mol, 10.0g), potassium sulfide (0.18 mol, 20.4g), dimethyl sulfoxide (300mL) sequentially into a 500mL round bottom flask, and stir the reaction at 140°C for 8h, thin-layer chromatography Monitor the reaction process until the reaction is complete; add water to quench the reaction, extract three times with 1.5L ethyl acetate, combine the organic phases, evaporate the ethyl acetate under reduced pressure, and recrystallize and purify with petroleum ether / ethyl acetate to obtain polysubstituted thiophene carboxaldehyde Compound IIa, yield 72%. The reaction equation is as follows:
[0039]
[0040] references
[0041] 1. (a). J. Cossy, N. Blanchard and C. Meyer, Tetrahedron Letters, 2002, 43, 1801-1805; (b). K. Sakaguchi, M. Fujita and Y. Ohfune, Tetrahedron Letters, 1998, 39, 4313-4316; (c). Y. Maeda, T. Nishimura and S. Uemura, Chemistry Lette...
Embodiment 2
[0056] The structure, NMR, and high-resolution mass spectrometry data of the product obtained in Example 2 are as follows:
[0057]
[0058] 1H NMR (400MHz, CDCl 3 ,ppm):δ=9.96(s,1H),7.80(d,J=1.2Hz,1H), 7.63(s,1H),7.30-7.25(m,2H),2.34(s,3H); 13 C NMR (100MHz, CDCl 3 , ppm): δ=183.0, 144.2, 143.6, 137.9, 134.7, 131.5, 129.7, 129.0, 126.1, 21.1; HRMS calcd for C 12 h 11 OS[M+H] + 203.0525; found: 203.0529.
Embodiment 3
[0059] The structure of embodiment 3 gained product, NMR, high-resolution mass spectrum data are as follows:
[0060]
[0061] 1 H NMR (400MHz, CDCl 3 ,ppm):δ=9.95(d,J=1.2Hz,1H),7.79(d,J=1.2Hz,1H),7.61(s,1H),7.18-7.16(d,J=8.0Hz,1H) ,7.11-7.09(d,J=8.0Hz,2H),2.35(s,3H),2.30(s,3H); 13 C NMR (100MHz, CDCl 3 , ppm): δ=183.0, 143.7, 143.4, 137.5, 135.6, 134.6, 132.3, 132.2, 130.6, 130.2, 128.8, 20.8, 20.1; HRMS calcd for C 13 h 13 OS[M+H] + 217.0682; found: 217.0687.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com