Water-soluble fullerene derivative with determined structure as well as preparation method and application thereof
A fullerene derivative, water-soluble technology, applied in the direction of fullerene, nano-carbon, etc., can solve the problems of difficult analysis, uncertain structure, difficult control of the introduction process of hydroxyl group, etc. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: C 60 Preparation of Fullerene Monoaddition Carboxylic Acid Derivatives
[0055] (1) Synthesis of di-tert-butyl bromomalonate
[0056] Di-tert-butyl malonate (200mg, 0.92mmol) was dissolved in tetrahydrofuran (100mL). After complete dissolution, 138μL (0.92mmol) of DBU (CAS accession number: 6674-22-2) was added to In the above solution, the reaction was then warmed to room temperature and stirring was continued for 1 h, after which the reaction was cooled to -78 °C and CBr 4 306mg (0.92mmol), continue to stir for 1.5h, after the reaction is over, use saturated NH 4 Cl solution was quenched, then added n-hexane for extraction, the organic layer was washed twice with saturated NaCl, the aqueous layer was extracted twice with dichloromethane, and washed twice with saturated NaCl, then the organic phase was collected and mixed with anhydrous magnesium sulfate After drying, filter the solution after drying and concentrate to obtain a yellow oily liquid; use di...
Embodiment 2
[0067] Embodiment 2: containing hydroxyl C 60 Preparation of Fullerene Carboxylic Acid Derivatives
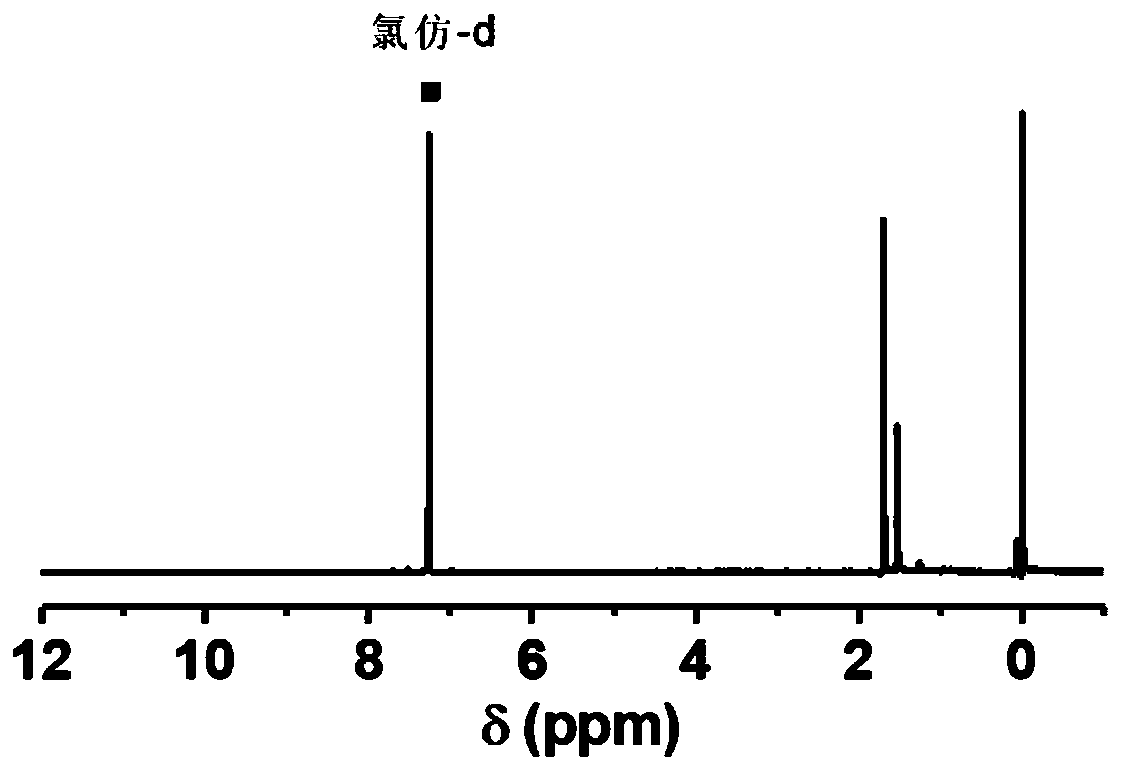
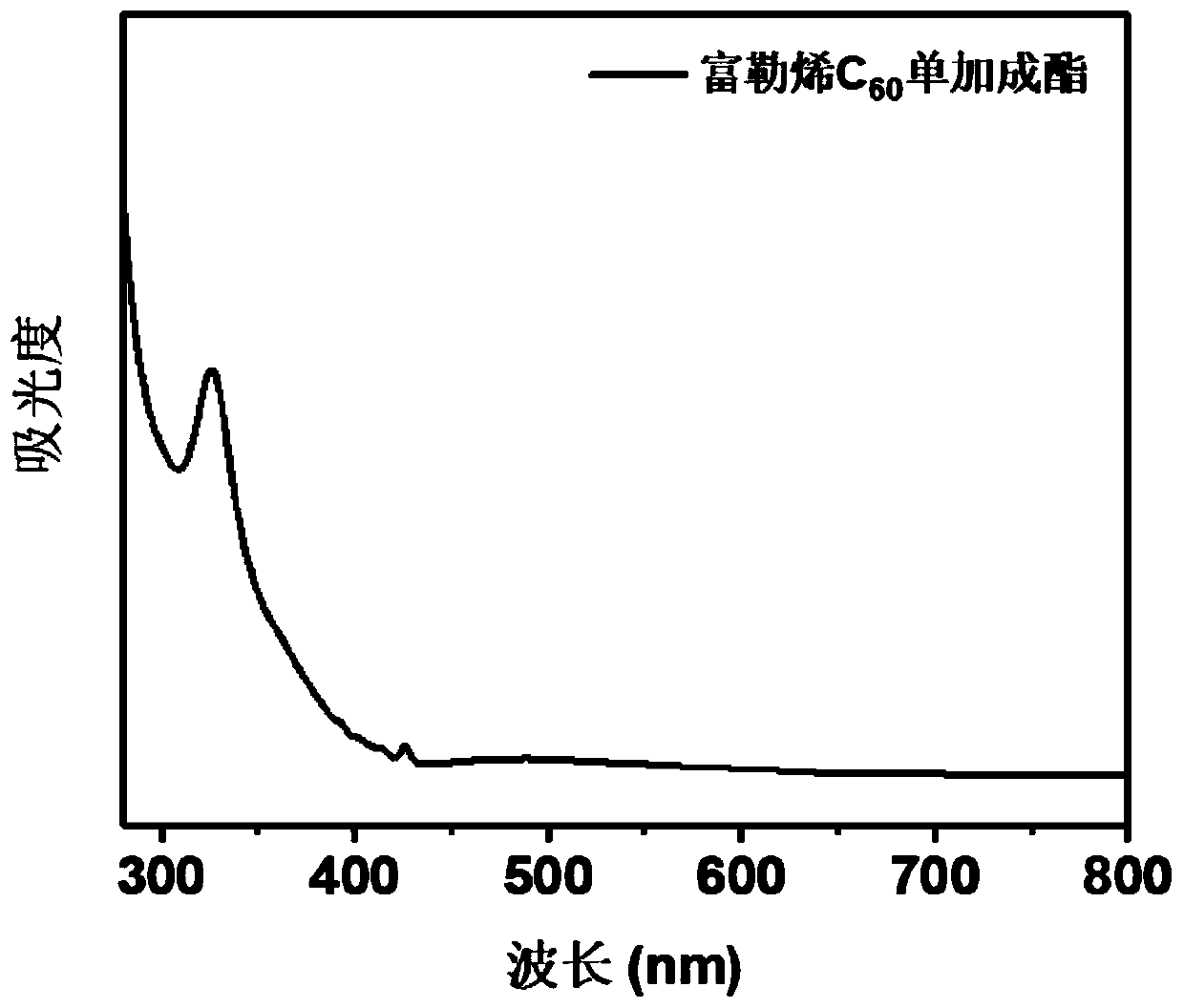
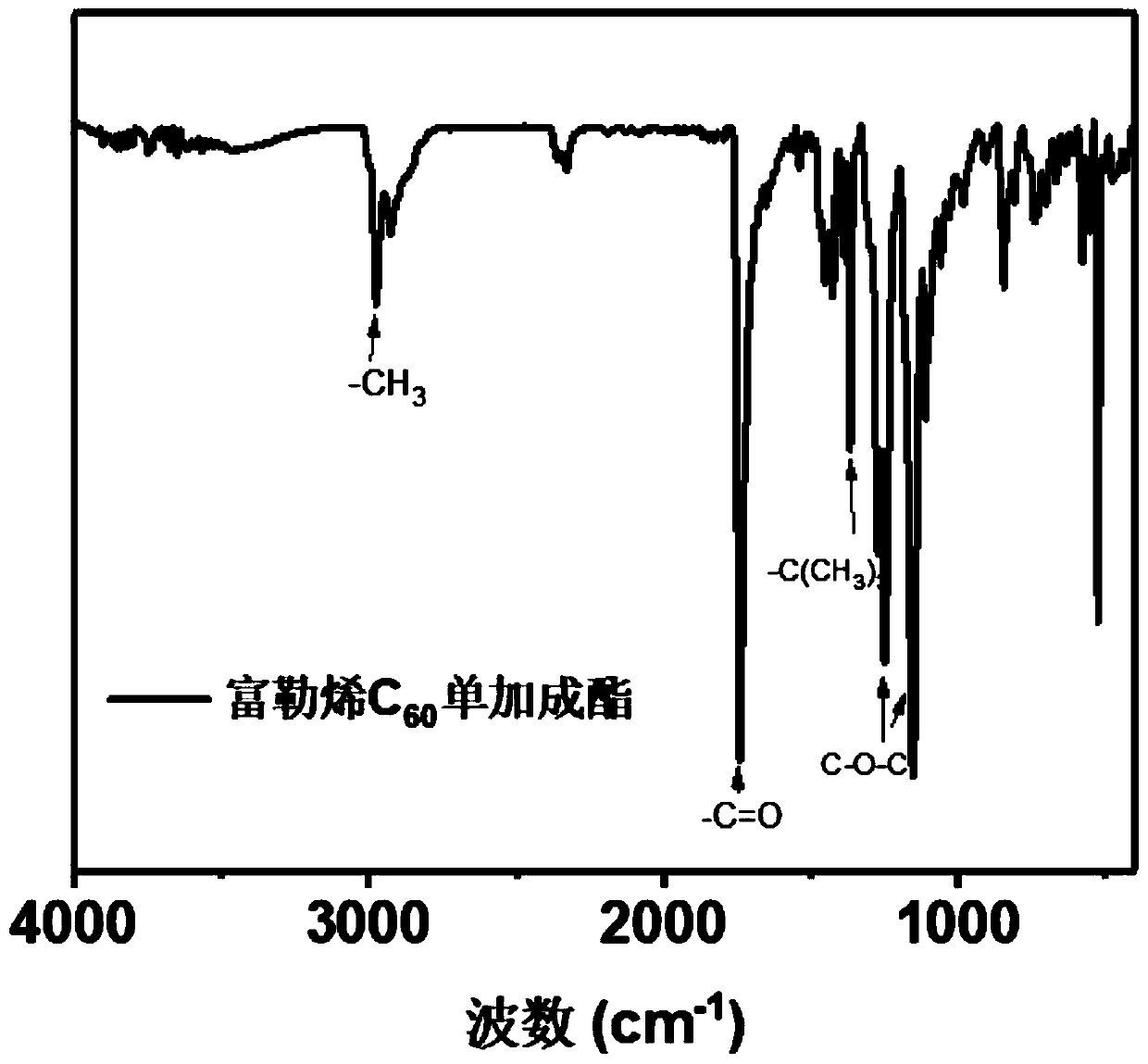
[0068] (1) Fullerene C 60 monoaddition ester
[0069] Fullerene C 60 (1g, 1.38mmol) was dissolved in o-xylene (500mL), ultrasonically dissolved completely, stirred at room temperature; 234uL (1.38mmol) of diethyl bromomalonate was dissolved in 3mL o-dichlorobenzene, and added dropwise to the above C 60 In o-dichlorobenzene solution; DBU 206uL (1.38mmol) was dissolved in 3mL o-dichlorobenzene, and added dropwise to the above C 60 In o-dichlorobenzene solution; stir at room temperature, and check the progress of the reaction by thin chromatography while stirring. After the reaction is complete, add 167uL glacial acetic acid to quench the reaction, spin the reaction solution to dryness, dissolve the solid in o-xylene, and use toluene as a rinse The reagent was separated by silica gel column chromatography, and the solid was dried overnight at 50°C.
[0070] (2)C 60 Preparat...
Embodiment 3
[0078] Example 3: metal fullerene Gd@C 82 Preparation of Monoaddition Carboxylic Acid Derivatives
[0079] (1) Metallofullerene Gd@C 82 preparation of
[0080] Gd@C 82 Molecules are passed through the arc discharge method in a DC arc discharge furnace ( method) synthesis.
[0081] (2) Metallofullerene Gd@C 82 -C s The Binger addition reaction, the steps include:
[0082] Under room temperature conditions, nitrogen gas was passed into a 50ml three-neck flask for 30min, then 2g of sodium hydride was added, 40ml of chromatographically pure toluene was added, after stirring for a period of time, 2ml of di-tert-butyl bromomalonate was added, and the solution was stirred until the solution was light yellow, then centrifuged to obtain off-white precipitate and light yellow solution, take 150ml Gd@C 82 The toluene solution was added into a 250ml three-necked flask, nitrogen gas was introduced, the above-mentioned light yellow solution was added dropwise, and stirred at room t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com