Copper-iridium composite oxide catalyst for preparing methanol by methane oxidation
A technology of composite oxides and catalysts, applied in metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, oxidation reaction preparation, etc., can solve the problems of poor catalyst activity and poor stability of active sites, etc. Achieve the effects of convenient operation, simple use conditions and high catalytic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
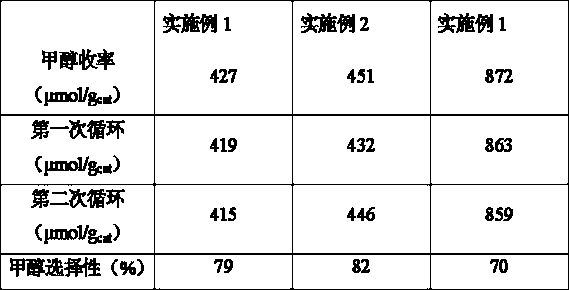
[0018] A kind of copper-iridium composite oxide catalyst that is used for methane oxidation to methanol, described catalyst is made of CuO and IrO 2 composition. The weight percentage of iridium is 1% based on 100% by mass of the catalyst. First, a certain concentration of copper acetate solution and sodium hydroxide solution were mixed, and then hydrothermally treated at 100 °C for 30 min to obtain a black CuO precipitate, which was centrifuged, washed, and dried to obtain CuO powder. Then use the equal volume impregnation method to impregnate the solution containing the required amount of chloroiridic acid onto the carrier CuO overnight, then dry it at 80 °C, and finally treat it under a nitrogen atmosphere at 500 °C for 4 hours to obtain a copper-iridium composite oxide catalyst.
Embodiment 2
[0020] A kind of copper-iridium composite oxide catalyst that is used for methane oxidation to methanol, described catalyst is made of CuO and IrO 2 composition. The catalyst is based on 100% by mass, and the weight percentage of iridium is 3%. Firstly, Cu-BTC was prepared, and then it was calcined in air at 500°C for 3 hours to obtain CuO support. Afterwards, the equal volume impregnation method was used to impregnate the solution containing the required amount of chloroiridic acid on the carrier CuO overnight, and then 80 o C drying, and finally calcined in an air atmosphere at 700 °C for 3 hours to prepare a copper-iridium composite oxide catalyst for methane oxidation to methanol.
Embodiment 3
[0022] A kind of copper-iridium composite oxide catalyst that is used for methane oxidation to methanol, described catalyst is made of CuO, ZnO and IrO 2 composition. Based on the weight of the catalyst being 100%, the weight percentage of iridium is 2.0%, and the weight percentage of ZnO is 10%. Cu-BTC was prepared first, and then it was calcined at 500 °C in air for 3 hours to obtain CuO support. Co-impregnate the required amount of chloroiridic acid solution and zinc acetate solution on the carrier CuO carrier overnight, then dry it at 100 °C, and finally bake it in an air atmosphere at 600 °C for 6 hours to prepare methanol for methane oxidation. Zinc-doped copper-iridium composite oxide catalyst.
[0023] Take 10 mg of the catalysts described in Examples 1-3 respectively, and place them in a high-pressure reactor (with a lining volume of 100 mL) for experimentation. The experimental conditions are as follows: 30 mL of water was added to the reactor, the methane pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com