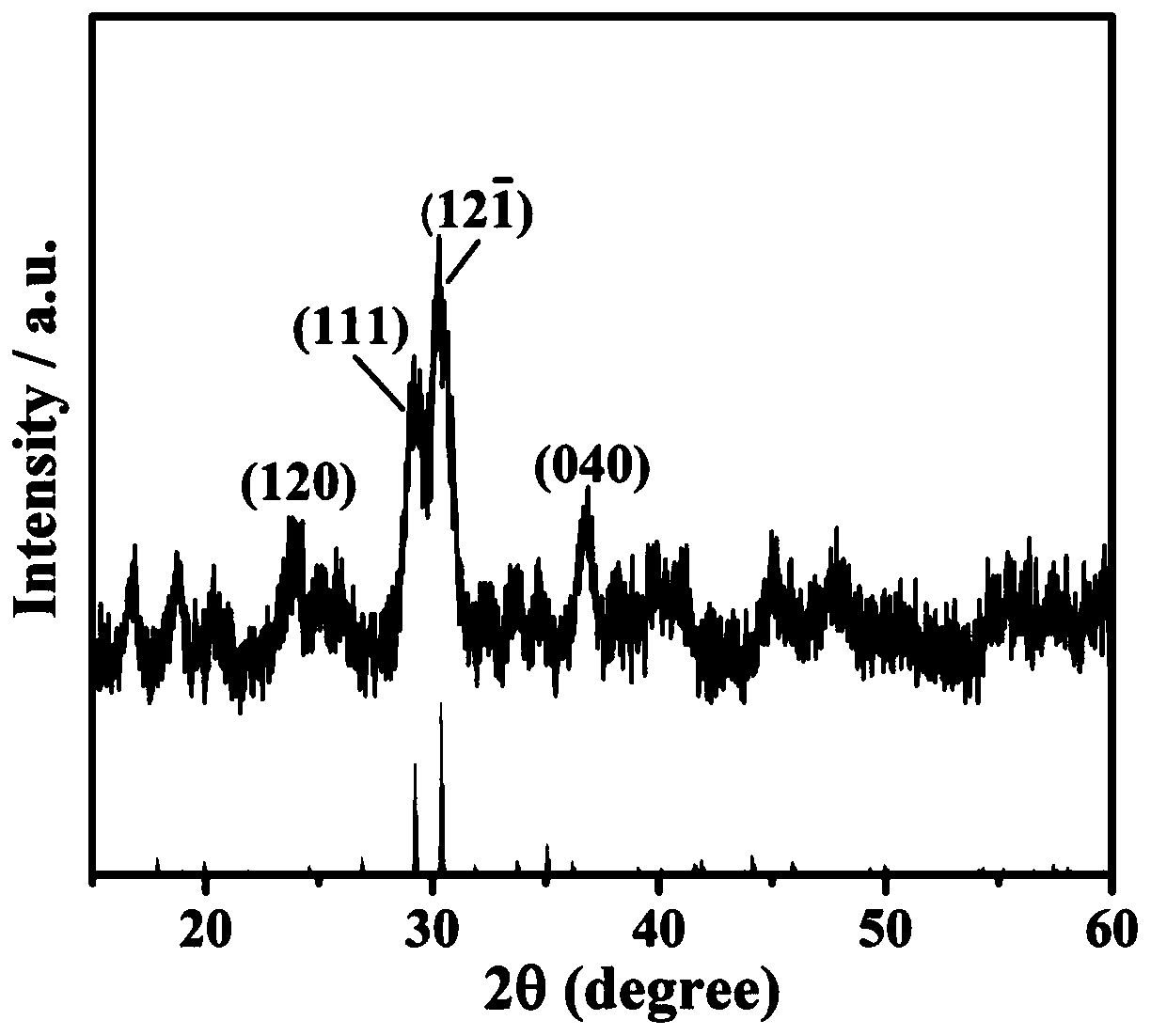
Application of cobalt pyrophosphate nanomaterial to construction of nitric oxide electrochemical sensors
A technology of cobalt pyrophosphate nanometer and nitric oxide, which is applied in the direction of material electrochemical variables, nanotechnology, nanotechnology, etc., can solve the problems of high cost and unfavorable large-scale production and use, and achieve long cycle life and easy commercialization Application, effect of high specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] To construct an electrochemical sensor for nitric oxide, assemble it as follows:
[0034] (1) Preparation of cobalt pyrophosphate nanomaterials
[0035] Mix the sodium pyrophosphate solution and the cobalt chloride solution to obtain a reaction solution. The molar ratio of sodium pyrophosphate and cobalt chloride in the reaction solution is 1.5. The reaction solution is stirred and reacted at 50°C for 10 hours and then heated at 8000r / min. Centrifuge at a speed of 10 min, take the precipitate, and use deionized water as the cleaning solution to centrifuge and wash the precipitate at a speed of 8000r / min, and then dry it at 60°C for 5 hours.
[0036] (2) Preparation of a working electrode coated with cobalt pyrophosphate nanomaterials
[0037] Disperse the cobalt pyrophosphate nanomaterial prepared in step (1) in deionized water at a ratio concentration of 3.3 mg / mL to obtain an electrode modification solution, and apply the electrode modification solution to a glassy c...
Embodiment 2
[0041] To construct an electrochemical sensor for nitric oxide, assemble it as follows:
[0042] (1) Preparation of cobalt pyrophosphate nanomaterials
[0043] Mix the sodium pyrophosphate solution and the cobalt acetate solution to obtain a reaction solution. The molar ratio of sodium pyrophosphate and cobalt acetate in the reaction solution is 1.7. Stir the reaction solution at 80°C for 12 hours and then react at a speed of 12000r / min. Centrifuge for 2 minutes, take the precipitate, use propanol as the cleaning solution, centrifuge and wash the precipitate at a speed of 12000r / min, and then dry it at 120°C for 0.5h;
[0044] (2) Preparation of a working electrode coated with cobalt pyrophosphate nanomaterials
[0045] Disperse the cobalt pyrophosphate nanomaterial prepared in step (1) in deionized water at a proportioning concentration of 5.0 mg / mL to obtain an electrode modification solution, and apply the electrode modification solution to a gold electrode, and heat Dry ...
Embodiment 3
[0049] To construct an electrochemical sensor for nitric oxide, assemble it as follows:
[0050] (1) Preparation of cobalt pyrophosphate nanomaterials
[0051] Mix potassium pyrophosphate solution and cobalt sulfate solution to obtain a reaction solution, the molar ratio of potassium pyrophosphate and cobalt sulfate in the reaction solution is 0.4, stir the reaction solution at 10°C for 16 hours and then at a speed of 3000r / min Centrifuge for 20 minutes, take the precipitate, use ethanol as the cleaning solution to centrifuge and wash the precipitate at a speed of 3000r / min, and then dry it at 30°C for 12 hours.
[0052] (2) Preparation of a working electrode coated with cobalt pyrophosphate nanomaterials
[0053]Disperse the cobalt pyrophosphate nanomaterial prepared in step (1) in deionized water at a proportioning concentration of 10 mg / mL to obtain an electrode modification solution, and apply the electrode modification solution to a screen-printed electrode at 20° C. Le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com