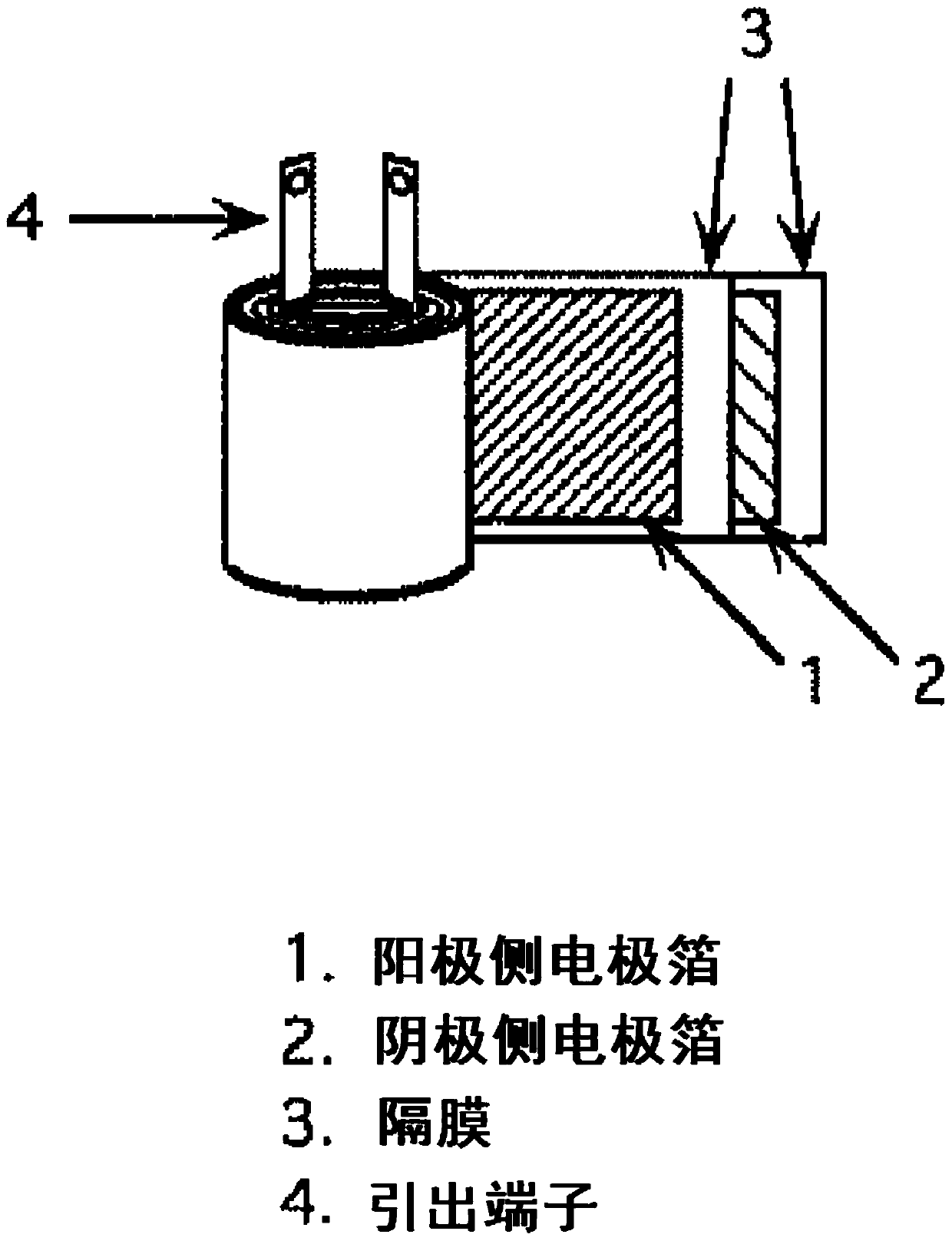
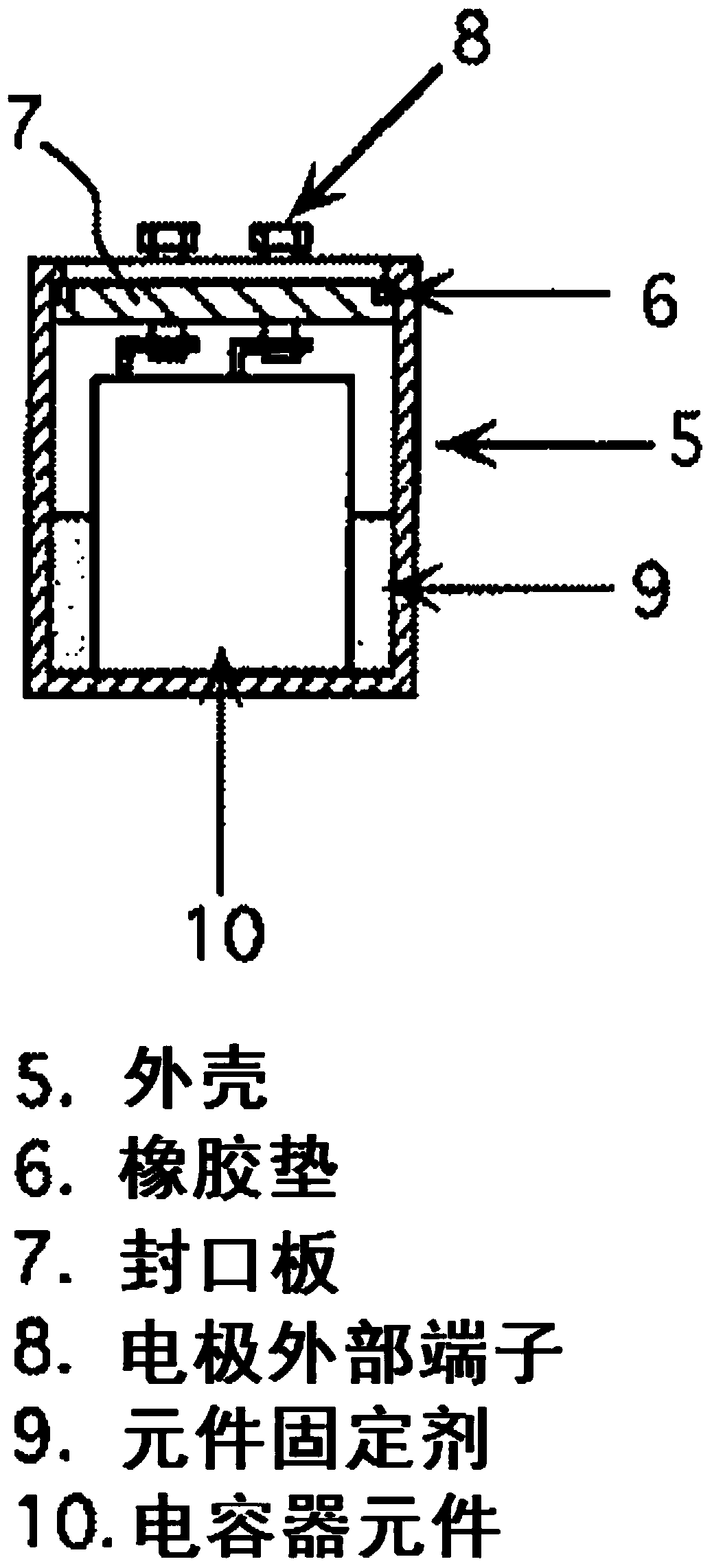
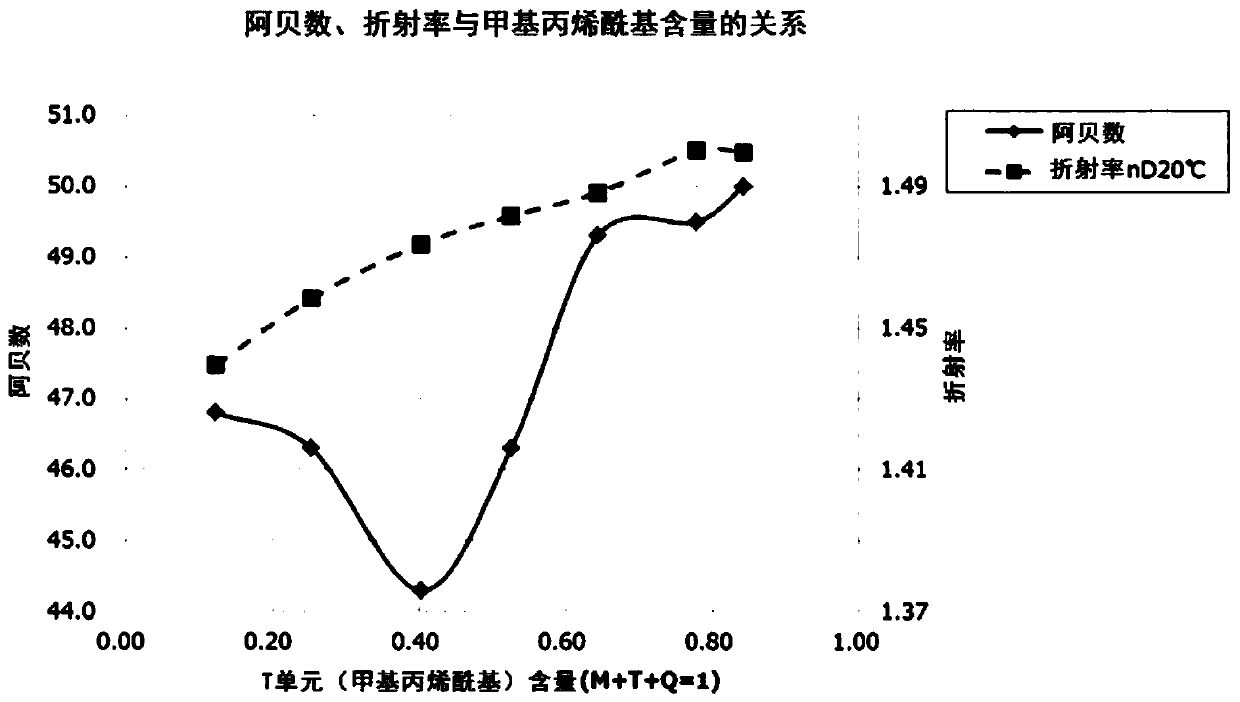
Polyorganosiloxane, polyorganosiloxane composition, cured product, polyorganosiloxane-containing electrolytic solution for electrolytic capacitor, and electrolytic capacitor using same
A polyorganosiloxane, silicon bonding technology, applied in the direction of electrolytic capacitors, capacitors, circuits, etc., can solve problems such as difficulty in developing electrolytic capacitors and reduction in withstand voltage of electrolytic capacitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0411] The present invention will be specifically described below through examples, but the present invention is not limited thereto. In addition, the materials used in the examples and the measurement methods of the evaluation items are as follows. Parts and % in Examples are based on mass unless otherwise specified.
[0412] [Evaluation I: Examples 1-1 to 1-5, Comparative Examples 1-1 to 1-6]
[0413] [test methods]
[0414] 1. Determination of the number of reactive functional groups in polyorganosiloxane
[0415] Weigh 50 mg of polyorganosiloxane as a measurement object, accurately weigh and add 15 mg of toluene as an internal standard. Further, add 1g of heavy chloroform to dissolve, use 400MHz 1 H-NMR (AL-400 manufactured by JEOL Ltd.) was measured by setting the relaxation delay (Relaxation Delay) to 20 seconds. From the ratio of the signal intensity of each component to the signal intensity of the internal standard toluene, and the weighed value, the content of al...
Embodiment 2-1~2-22
[0519] [Evaluation II: Examples 2-1 to 2-22, Comparative Examples 2-1 to 2-11]
[0520] [test methods]
[0521] 1. Determination of the number of reactive functional groups in polyorganosiloxane
[0522] The measurement of the number of reactive functional groups in the polyorganosiloxane was performed by the same method as above.
[0523] 2. 29 Measuring method of Si-NMR
[0524] By the same method as above, carry out 29 Determination of Si-NMR.
[0525] 3. Determination of molecular weight
[0526] The molecular weight was measured by the same method as above.
[0527] 4. Determination of Refractive Index
[0528] 4-1. Refractive index measurement of a liquid sample before curing: The refractive index at the sodium D line wavelength was measured at 20° C. using an automatic refractometer (Refractometer RX-7000α) manufactured by Atago Corporation.
[0529] 4-2. Refractive index measurement of cured product:
[0530] Measuring method 1. Using multi-wavelength Abbe ref...
Synthetic example 2-1
[0587] [Synthesis Example 2-1] Synthesis method of polyorganosiloxane
[0588] As polyorganosiloxane raw materials, 22 g of methyl silicate MS51 manufactured by Mitsubishi Chemical Corporation, 263 g of 3-methacryloxypropyltrimethoxysilane KBM503 manufactured by Shin-Etsu Chemical Co., Ltd., and 56 g of Hexamethasone manufactured by Nusil Technology Co., Ltd. were used. Hydrolytic condensation was carried out while maintaining 15°C to 40°C using 170 g of toluene and 170 g of methanol as a solvent, a mixture of 81 g of 1N hydrochloric acid and 81 g of methanol as a catalyst, and water. Thereafter, after removing hydrochloric acid by desalination, the solvent and moisture were removed, and 221 g of the target liquid polyorganosiloxane was obtained by filtration.
[0589] Although using a common method and an inexpensive raw material, it can be obtained at a high yield of 65% by mass relative to the total amount of the above-mentioned polyorganosiloxane raw materials, and variabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com