Umbilical cord mesenchymal stem cell injection for treating systemic sclerosis accompanied by acroscleriasis and preparation method thereof
A systemic sclerosis and stromal cell technology, applied in systemic sclerosis, umbilical cord mesenchymal stem cell injection and its preparation field, can solve the problem of immature and unsuitable skin mesenchymal stem cell preparation and quality control. Clinical application and other issues, to achieve the effect of relieving acrosclerosis symptoms, long-term validity, and easy to use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A preparation method for umbilical cord mesenchymal stem cell injection, comprising the steps of:
[0045] S1. Prepare umbilical cord mesenchymal stem cells. The specific operation is as follows: S11. Collect umbilical cord specimens and perform cell separation within 48 hours;
[0046] S12. Use the tissue block inoculation method to isolate and cultivate primary cells, and use serum-free and xeno-free medium (StemMACS) for primary cell culture and subsequent cell expansion. TM MSC Expansion Media Kit XF, Miltenyi Biotec);
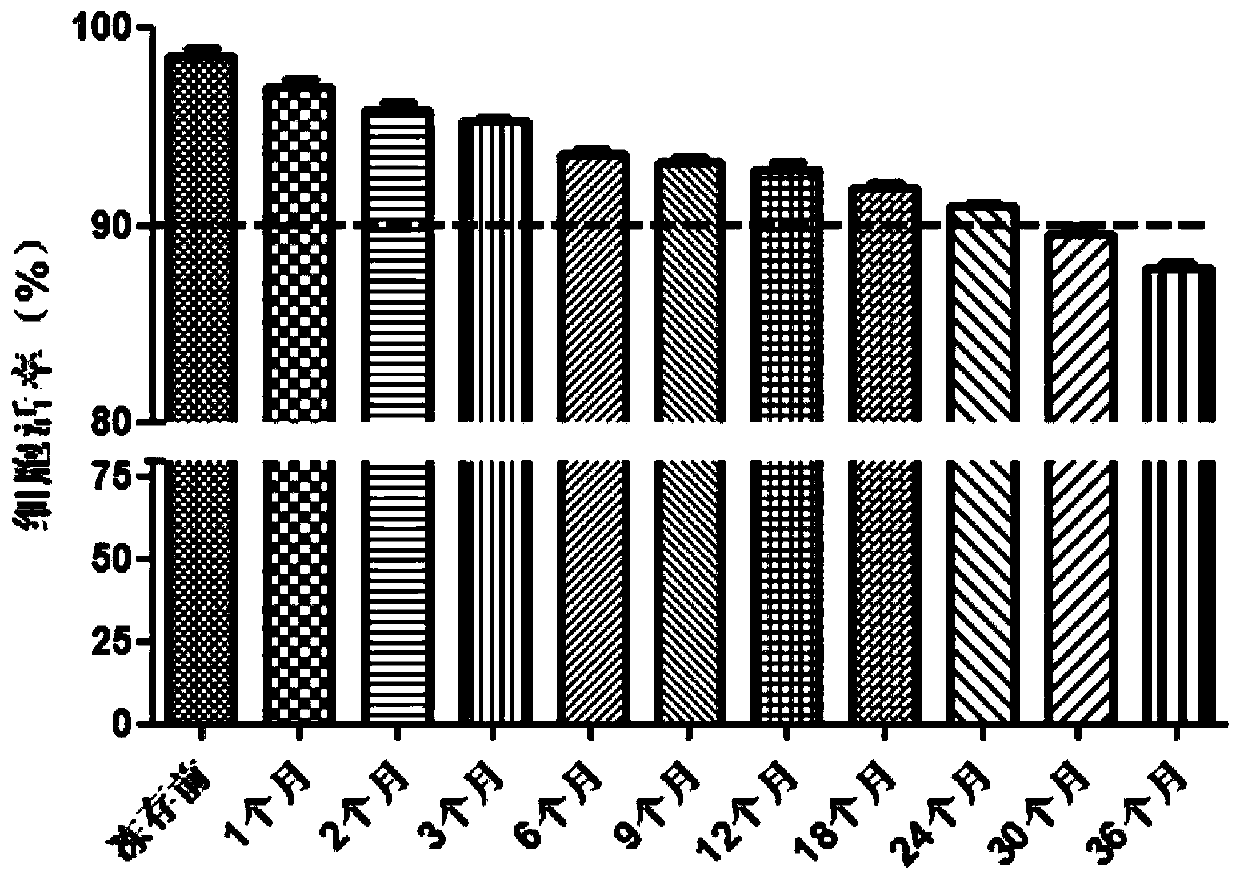
[0047] S13. Harvest the primary cells P0, subculture and expand to P1, and the cell passage density is 6000cell / cm2 , Harvest the cells in about 3 days; continue to subculture and expand to P2, harvest the P2 generation cells in about 3 days, and perform quality inspection. The inspection items include: cell viability, cell phenotype, cell differentiation potential, biological function, microorganisms, mycoplasma, Viruses and endotoxins; cells are ...
Embodiment 2
[0067] Preclinical research on the treatment of scleroderma mouse models with umbilical cord mesenchymal stem cells, the specific operations are as follows:
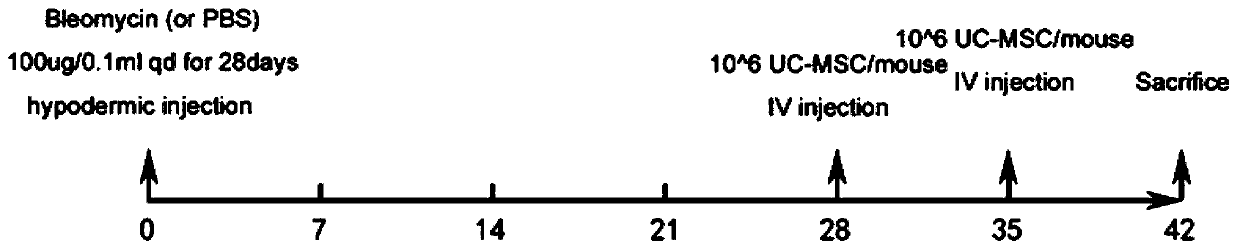
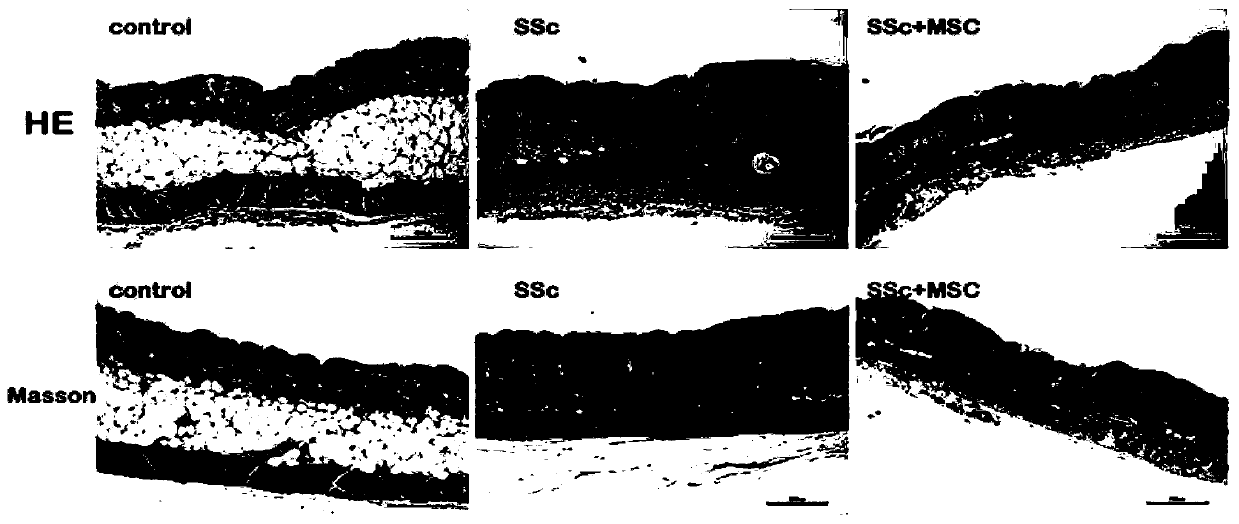
[0068] Establishment of scleroderma mouse model: (1) 30 female 6-week-old BALB / c mice were randomly divided into three groups: A, B, and C, respectively SSc model group, SSc model + mesenchymal stem cell treatment group, PBS control group; (2) Select the skin on the back of the mouse near the base of the tail, and remove the local hair of 1.5cm×1.5cm with depilatory cream; A, B two groups of mice were subcutaneously injected with 100 μL 1mg / ml bleomycin on the back, C group mice 100 μL PBS was subcutaneously injected on the back, once a day for 4 consecutive weeks; (3) Observe the changes of the skin at the injection site. model successfully.
[0069] Tail vein injection of umbilical cord mesenchymal stem cells to treat scleroderma mouse model: (1) After successful modeling, the umbilical cord mesenchymal stem cells pre...
Embodiment 3
[0073] Clinical research on the treatment of systemic sclerosis with umbilical cord mesenchymal stem cell injection, the specific operations are as follows:
[0074] 1. From May to November 2015, 42 outpatients and inpatients with systemic sclerosis were enrolled; the volunteers were randomly divided into two groups: traditional treatment group and combined treatment group. The general condition of the patients is shown in Table 2 below. Among them, MRSS: modified Rodnan score; dcSSc: diffuse scleroderma; lcSSc: localized scleroderma; ESSG index: activity index of European scleroderma study group. Through the statistics of gender, age, disease duration, MRSS, dcSSC / lcSSc and ESSG index and other indicators, the results showed that there was no significant difference between the traditional treatment group and the combined treatment group.
[0075] 2. Inclusion criteria: (1) Patients who understand and sign the informed consent form; (2) have not used any blood products (excep...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap