Antibody conjugate, preparation method and application thereof
An antibody conjugate and antibody technology, applied in the field of biomedicine, can solve the problems of CD30 positive tumor drug resistance and high cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1 Construction and expression of anti-human CD30 antibody cAC10
[0069] Synthesize DNA fragments encoding cAC10 monoclonal antibody heavy chain and light chain from the whole gene (see patent US7090843, B1RECOMBINANT ANTI-CD30ANTIBODIES AND USES THEREOF, Seattle Genetics, Inc., 2006, SEQ ID NO: 1 and SEQ ID NO: 9; and International Nonproprietary Names for Pharmaceutical Substances (INN).WHO Drug Information Vol.24, No.2, 2010), respectively cloned into the pEE12.4 eukaryotic expression vector of Lonza Company, and the operations of enzyme digestion and ligation were carried out according to commercial reagents Kit (DNALigation kit Ver2.0, TAKARA) instructions.
[0070] The constructed heavy chain and light chain expression vectors were respectively transformed into Escherichia coli DH5α, and positive clones were picked and inoculated in 500ml LB medium for amplification. DNA was extracted and purified using Qiagen's Ultrapure Plasmid DNA Purification Kit (Ult...
Embodiment 2
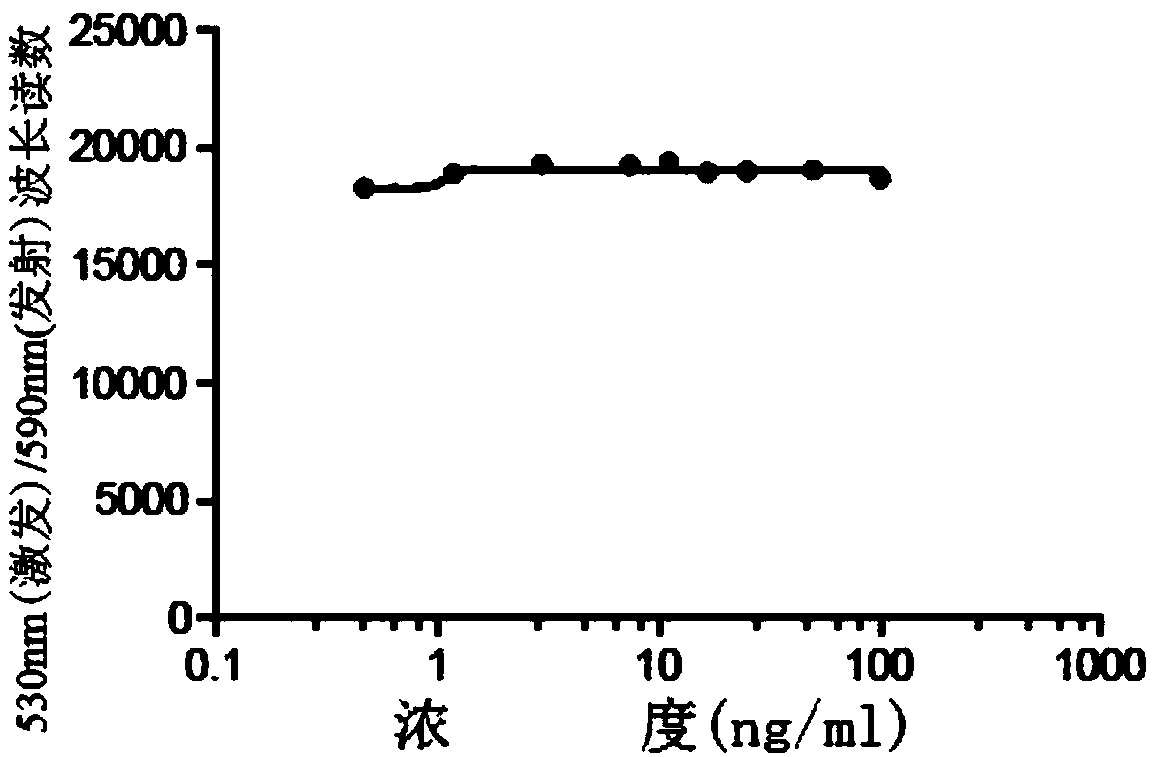
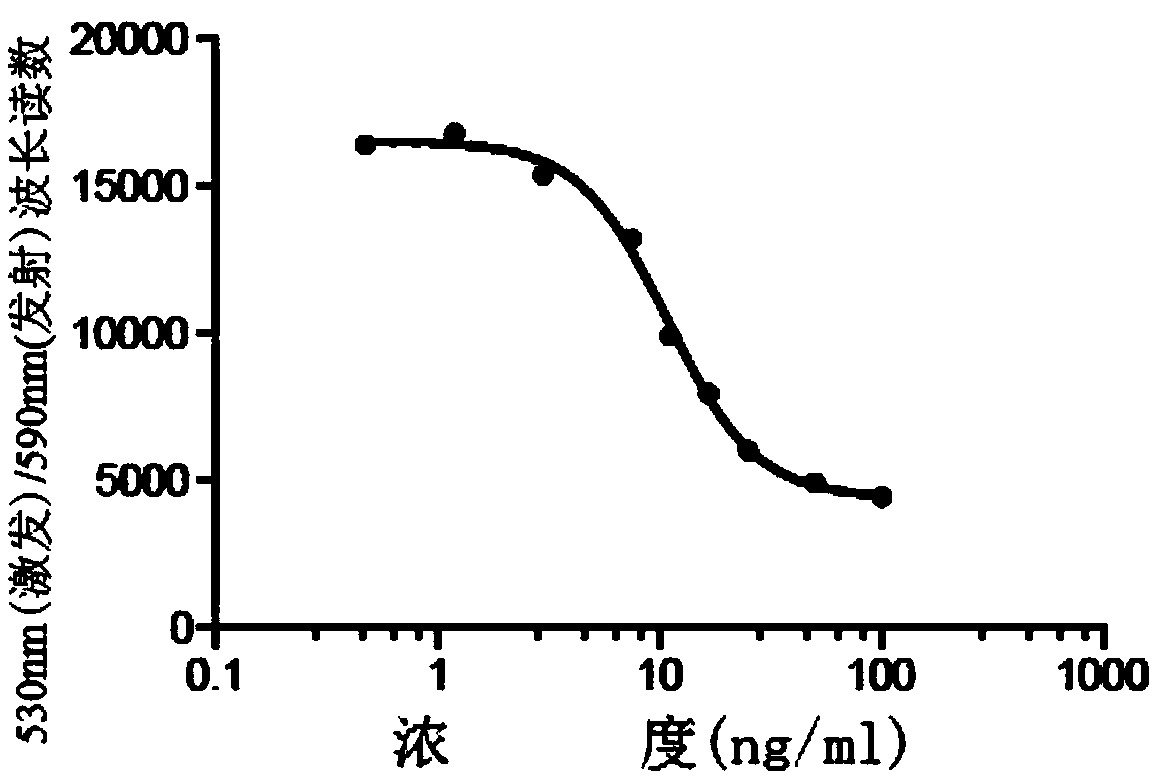
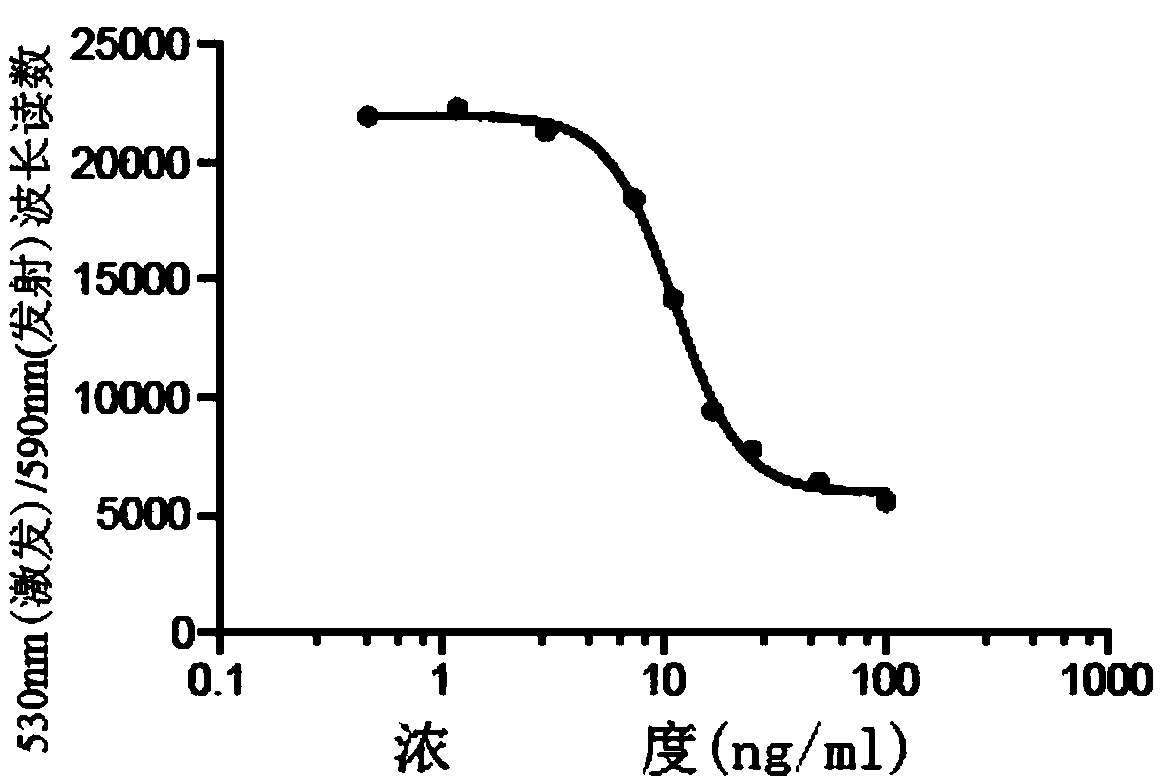
[0073] Embodiment 2 DAR detection method
[0074] The detection of DAR is based on the UV absorption of Ab and DM1 and the calculation of the coupling degree. The average number of DM1 linked to each antibody molecule is determined by measuring the absorbance at 252nm and 280nm. Ultraviolet / visible spectrophotometry (UV / Vis) is a simple and convenient method for determining protein concentration, as well as the average number of antibody-drug conjugates in an antibody drug-conjugate (ADC). DAR can be determined by using ADC absorbance measurements, and the extinction coefficients of the corresponding antibodies and drugs.
[0075] When the absorption coefficient of a certain pure substance under certain conditions is known, the test sample can be made into a solution under the same conditions, and its absorbance can be measured, and the content of the substance can be calculated by the following formula: A=E×c×l, Where A is the absorbance, E is the absorption coefficient, c i...
Embodiment 3
[0086] The preparation of embodiment 3 antibody conjugates
[0087] The invention guarantees the stability of the average coupling ratio (DAR) of the poison antibody by controlling the feeding ratio, pH, temperature, stirring, etc. of the coupling reaction.
[0088] A. Linker SMCC modified antibody:
[0089] Configuration buffer: 50 mM dipotassium hydrogen phosphate-potassium dihydrogen phosphate, 50 mM NaCl, 2 mM EDTA, pH 6.5. The antibody buffer was replaced by 10 times volume ultrafiltration, the final concentration of the antibody was 10 mg / mL, and argon gas was added to fill it. 1.5 ml of 20 mM SMCC (dissolved in DMSO or DMA) was added to 20 ml of cAC10 monoclonal antibody (Brentuximab, namely butuximab) solution, and reacted at room temperature for 4 hours. The reaction mixture was filtered through a Sephadex G25 gel column equilibrated with 5 column volumes of buffer. Collect the characteristic peaks of the antibody at OD280 to obtain the antibody modified by SMCC. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com