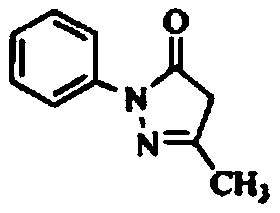
Edaravone sodium chloride injection liquid and preparation method thereof
A technology of edaravone sodium chloride and injection, which is applied in the direction of active ingredients of alkali/alkaline earth metal chlorides, pharmaceutical formulas, medical preparations of non-active ingredients, etc., and can solve the problem of uneven stability and influence on drug use Safety, long-term medication and other issues to achieve the effect of avoiding the generation of harmful impurities, improving the safety and compliance of medication, and reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
[0042] Preparation:
[0043] (1) Weigh 20% of the prescribed amount of water for injection, keep it warm at 20-30°C, fill the liquid with nitrogen for 15 minutes, add the prescribed amount of propylene glycol, stir to dissolve vitamin C, add edaravone and stir to dissolve, add sodium chloride and stir Dissolve, add 70% water for injection, add sodium carbonate to adjust the pH to 3.5;
[0044] (2) Constant volume, fine filtration through 0.22 μm filter membrane;
[0045] (3) Divide the obtained medicinal solution into 100ml / bottles, fill with nitrogen, stopper, cover, and sterilize with damp heat at 126°C for 6 minutes to obtain the finished product.
Embodiment 2
[0047]
[0048] Preparation:
[0049] (1) Weigh 20% of the prescribed amount of water for injection, keep it warm at 20-30°C, fill the liquid with nitrogen for 15 minutes, add the prescribed amount of glycerin, stir to dissolve vitamin C, add edaravone and stir to dissolve, add sodium chloride and stir Dissolve, add 70% water for injection, add sodium bicarbonate to adjust the pH to 5.0;
[0050] (2) Constant volume, fine filtration through 0.22 μm filter membrane;
[0051] (3) Divide the obtained medicinal solution into 100ml / bottles, fill with nitrogen, stopper, cover, and sterilize with damp heat at 126°C for 8 minutes to obtain the finished product.
Embodiment 3
[0053]
[0054]
[0055] Preparation:
[0056] (1) Weigh 20% of the prescribed amount of water for injection, keep it warm at 20-30°C, fill the liquid with nitrogen for 15 minutes, add the prescribed amount of propylene glycol, glycerin, vitamin C and stir to dissolve, add edaravone and stir to dissolve, add chloride Stir and dissolve the sodium, add 70% water for injection, add sodium bicarbonate and sodium carbonate to adjust the pH to 4.5;
[0057] (2) Constant volume, fine filtration through 0.22 μm filter membrane;
[0058] (3) Divide the obtained medicinal solution into 100ml / bottles, fill with nitrogen, stopper, cover, and sterilize with damp heat at 126°C for 6 minutes to obtain the finished product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com