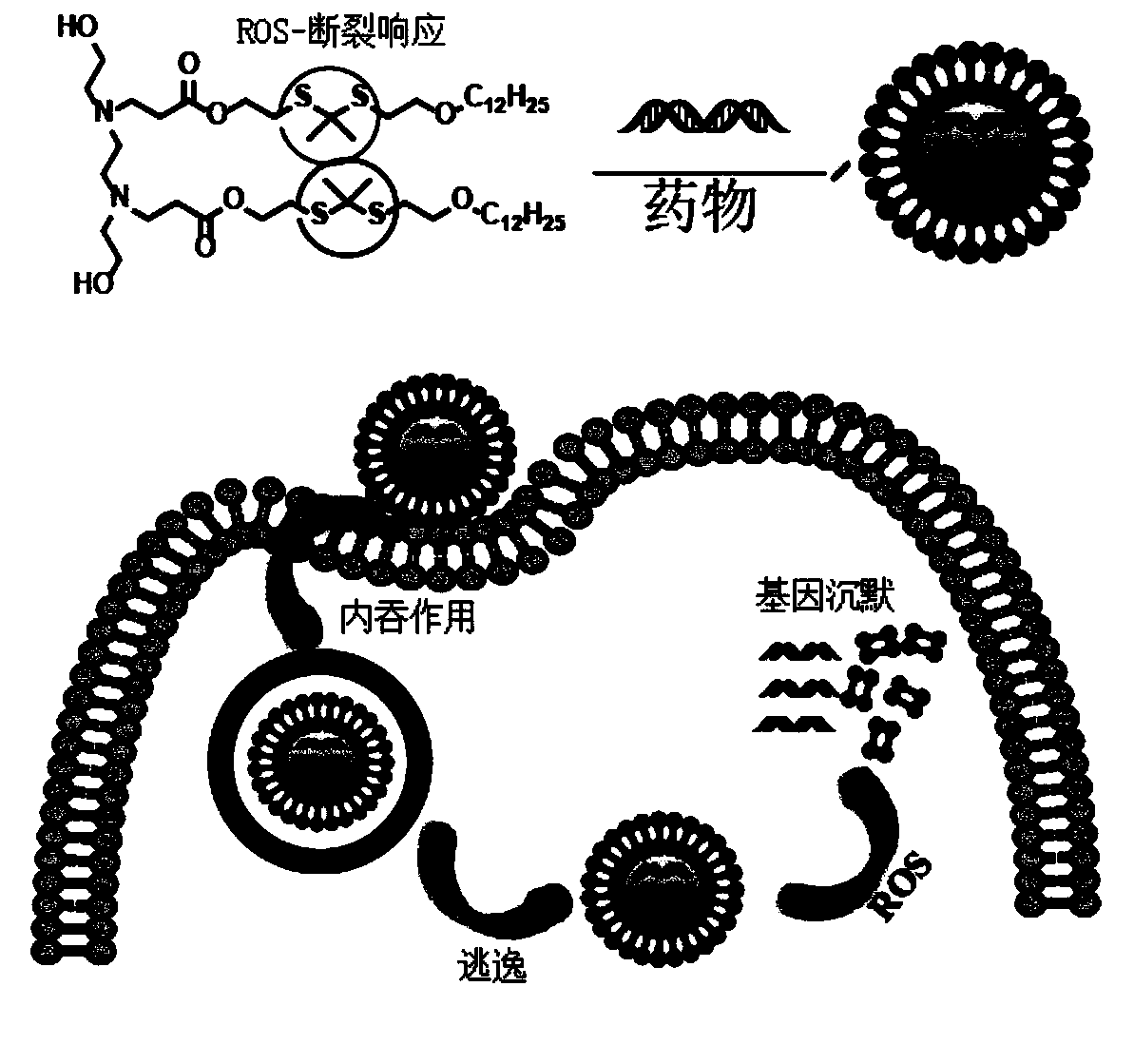
Nano-drug carrier and preparation method and application thereof
A nano drug and drug activity technology, applied in the field of biochemistry, can solve the problems of difficult drug release function, poor cell selectivity, affecting transfection efficiency, etc., and achieve the effects of reducing drug toxicity, high delivery efficiency, and improving release efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1 prepares compound b
[0061]
[0062] Dissolve 1 mM compound a in 3 ml of 1,4-dioxane solvent, add 1 mM potassium hydroxide powder, then add 1 mM n-dodecyl bromide, raise the temperature to 105° C., and react overnight. TLC monitoring, after the reaction was completed, it was extracted with dichloromethane and water, dried, rotary evaporated, and passed through the column with petroleum ether and ethyl acetate 5:1 to obtain the product, namely compound b.
Embodiment 2
[0063] Embodiment 2 prepares formula I-1 compound
[0064]
[0065] Dissolve compound b in anhydrous dichloromethane and add 1.2 equivalents of triethylamine, add acryloyl chloride dropwise under ice bath conditions, remove the ice bath, react at room temperature for 5 hours, add water to quench after the reaction is completed, extract, dry, rotary evaporate, and use Petroleum ether and ethyl acetate 10:1 through the column. A light yellow liquid is obtained, namely the compound of formula I-1.
Embodiment 3
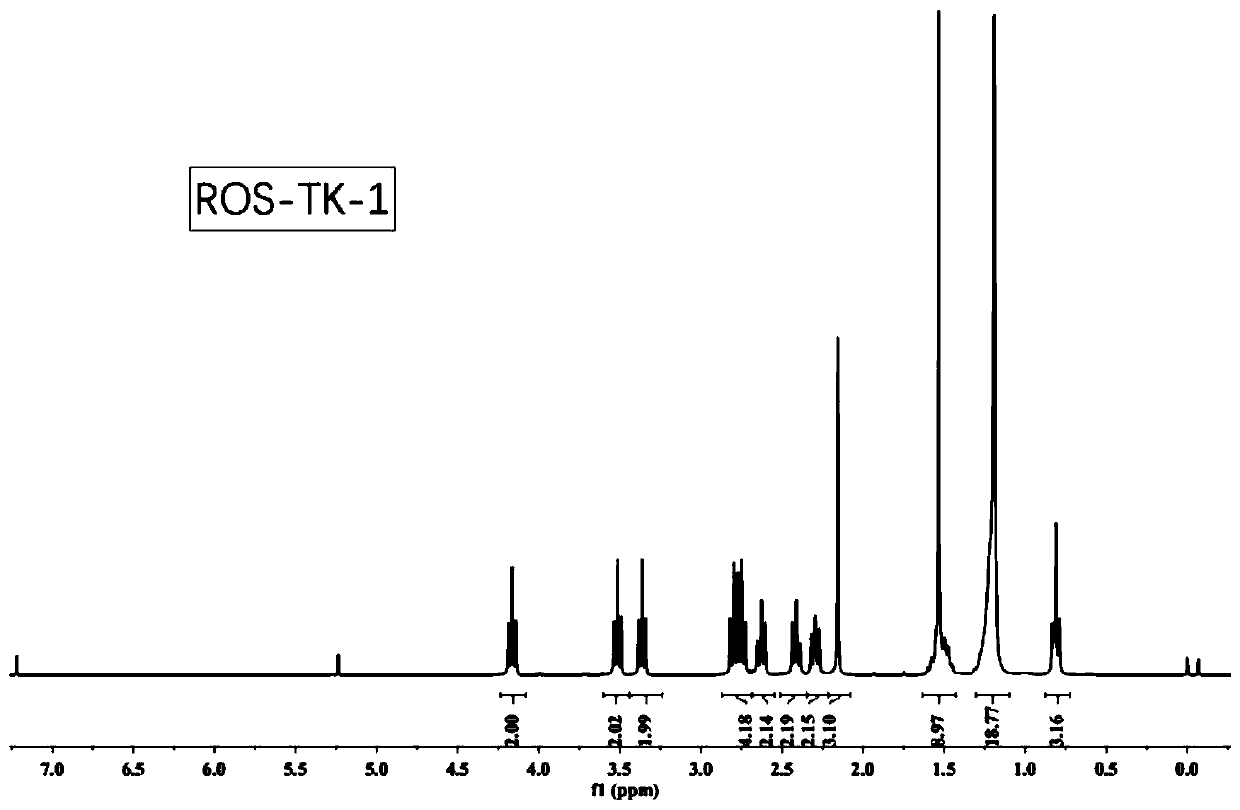
[0066] Embodiment 3 preparation formula II-1 liposome: ROS-TK-1
[0067]
[0068] Mix the compound of formula I-1 with the fatty amine shown in compound 1 at a molar ratio of 2.4:1, heat to 80°C, react for 36 hours, and pass through the column with dichloromethane and methanol at 20:1 to prepare liposomes of formula II-1 : ROS-TK-1, its nuclear magnetic spectrum is as follows figure 2 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com