Method for removing arsenic from acidic arsenic-containing solution by using iron salt
A solution and acidic technology, applied in chemical instruments and methods, iron compounds, neutralized water/sewage treatment, etc., can solve the problems of long precipitation reaction time, harsh reaction conditions, and difficult access to scorodite, etc., to achieve accelerated growth, The effect of mild process conditions and enhanced mutual repulsion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
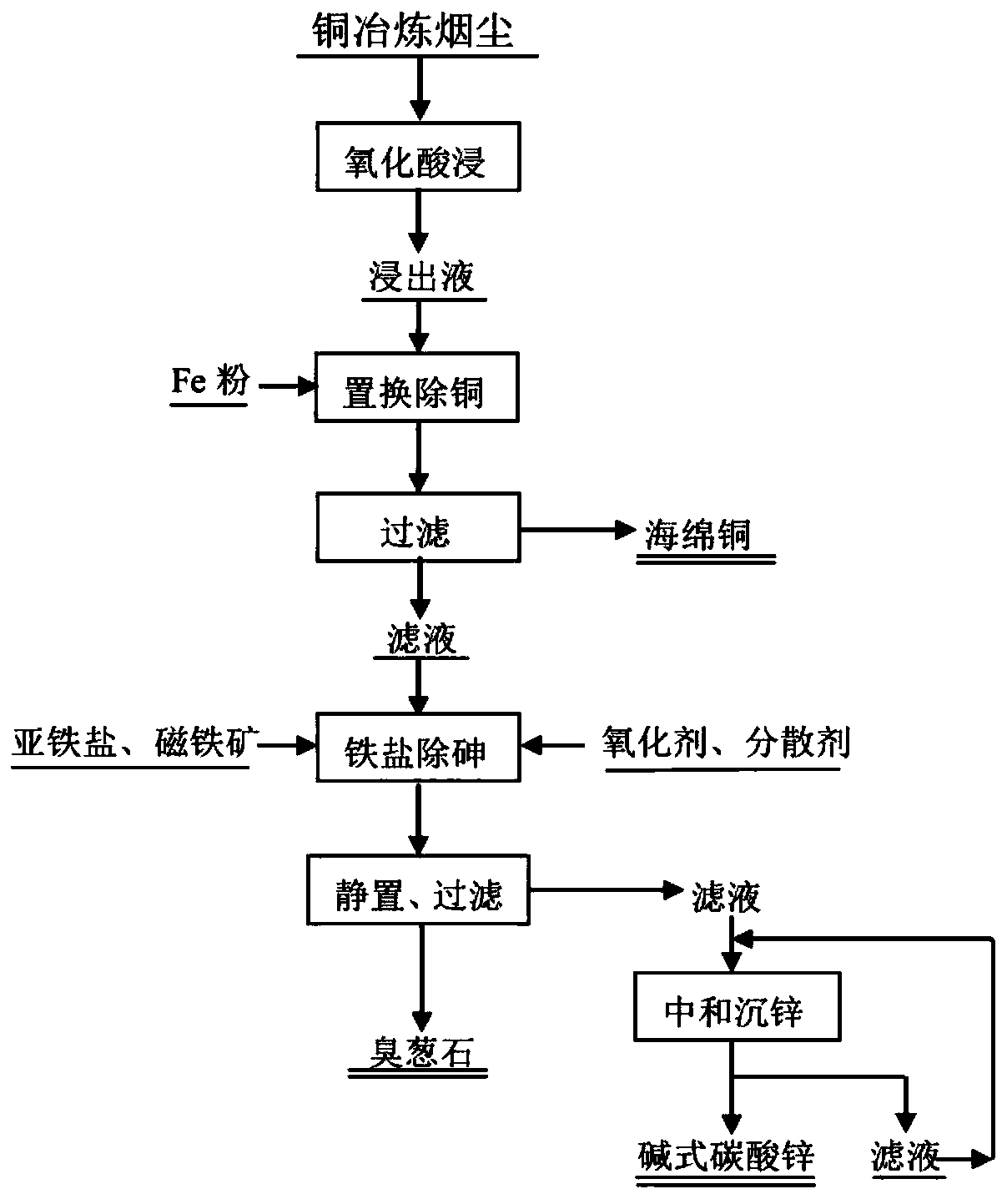
[0034] Such as figure 1 Shown, a kind of method that utilizes iron salt to remove arsenic from acidic arsenic-containing solution (copper smelting soot pickling solution), acid is sulfuric acid in the copper smelting soot pickling solution in this example, copper smelting soot pickling solution main component ( g / L) is Cu 22.409, As22.830, Fe 0.242, Zn 5.498, Cd 2.735. The method of this embodiment specifically includes the following steps:
[0035] (1) with the step (1) of comparative example;
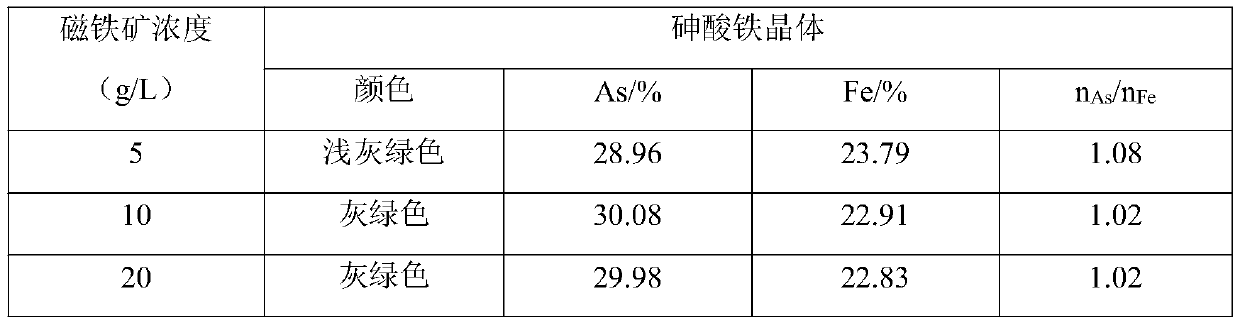
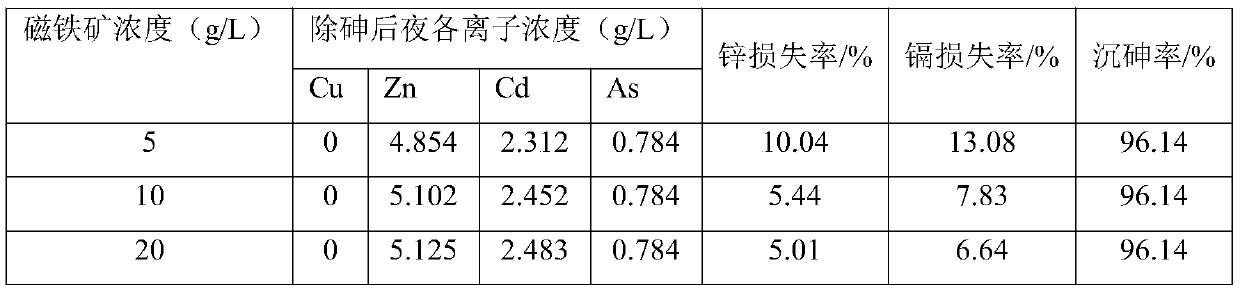
[0036] (2) Take 200mL copper-removed solution and put them in three beakers for arsenic removal test, adjust the pH to 2.0 with sodium carbonate, add ferrous sulfate according to the total Fe / As molar ratio in the solution 1.2:1, stir and heat to 30 ℃, add magnetite powder, control the concentration of magnetite in the solution to 5, 10, 20g / L respectively, add oxidant ammonium persulfate, control the concentration of oxidant in the solution to 1mol / L. After stirring for 5-10min, a...
Embodiment 2
[0044] A kind of method that utilizes iron salt to remove arsenic from acidic arsenic-containing solution (copper smelting smoke pickling solution), in this example, the acid in the copper smelting smoke pickling solution is sulfuric acid, and the main component of copper smelting smoke pickling solution (g / L ) is Cu 22.409, As 22.830, Fe0.242, Zn 5.498, Cd 2.735. The method of this embodiment specifically includes the following steps:
[0045] (1) with the step (1) of comparative example;
[0046] (2) Take three 200mL copper-removed solutions for arsenic removal test, adjust the pH to 2.0 with sodium carbonate, according to the total Fe / As molar ratio in the solution (1.0:1), (1.2:1), (1.5:1 ) add ferrous sulfate, stir and heat to 30°C, add magnetite powder, control the concentration of magnetite in the solution to be 10g / L, add oxidant ammonium persulfate, control the concentration of oxidant in the solution to be 1mol / L, After stirring for 5-10min, add the dispersant sodi...
Embodiment 3
[0052] A kind of method that utilizes iron salt to remove arsenic from acidic arsenic-containing solution (copper smelting smoke pickling solution), in this example, the acid in the copper smelting smoke pickling solution is sulfuric acid, and the main component of copper smelting smoke pickling solution (g / L ) is Cu 22.409, As 22.830, Fe0.242, Zn 5.498, Cd 2.735. The method of this embodiment specifically includes the following steps:
[0053] (1) with the step (1) of comparative example;
[0054] (2) Take three 200mL copper-removed solutions for arsenic removal test, adjust the pH to 2.0 with sodium carbonate, add ferrous sulfate according to the Fe / As molar ratio of 1.2:1, stir and heat to 30°C, and add magnetite powder , control the concentration of magnetite in the solution to be 10g / L, add oxidant hydrogen peroxide, ferrate, ammonium persulfate respectively, control the concentration of oxidant in the solution to be 1mol / L. After stirring for 5-10min, add the dispersan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com