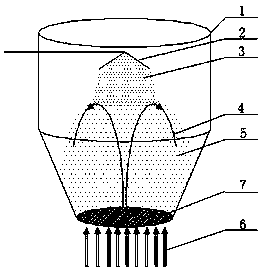
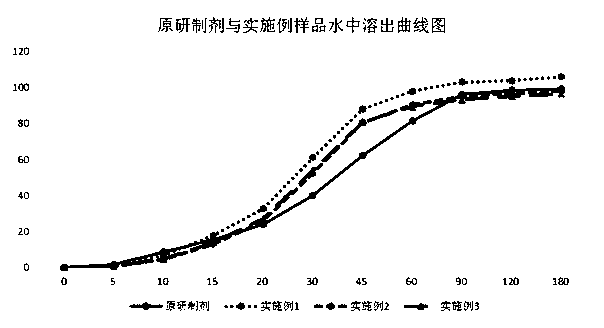
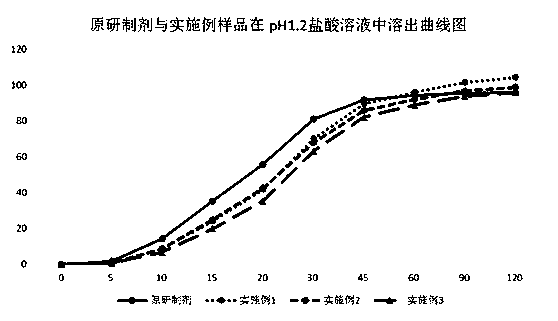
Indapamide capsule preparation method
A technology of indapamide and capsules, which is applied in the field of preparation of indapamide capsules, can solve the problems of high-temperature adhesives that are unfavorable to production personnel for mass production operations, hidden dangers of production personnel's GMP environmental safety, pollution of the environment and operators, etc. , to achieve the effect of reducing the influence of manual operation, shortening the preparation time, and reducing production safety hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of Indapamide Capsules (Specification: 2.5 mg)
[0044]
[0045] Preparation of granulation solution: Dissolve the prescribed amount of hypromellose E5 in ethanol aqueous solution, make HPMC-ethanol aqueous solution, stir to dissolve, then put the prescribed amount of indapamide into HPMC-ethanol aqueous solution, stir well to a lump-free suspension.
[0046] 2. Granulation: Put the weighed cornstarch, lactose, and povidone K90 into the fluidized bed in sequence, and use top spray granulation. The parameters set in the fluidized bed at different times are shown in the table below:
[0047]
[0048] After spraying the granulation liquid on the top, it is dried, and the drying parameters are set: the material temperature is 40°C, the air inlet temperature is 55°C, and the air volume of the fan is 600m 3 / h. Moisture is controlled below 3.0%.
[0049] 3. Total mixing: Put the 40-mesh sieved granules and the prescribed amount of silicon dioxid...
Embodiment 2
[0051] Example 2 Preparation of Indapamide Capsules (Specification: 2.5 mg)
[0052]
[0053] Preparation of granulation solution: Dissolve the prescribed amount of hypromellose E5 in ethanol aqueous solution, make HPMC-ethanol aqueous solution, stir to dissolve, then put the prescribed amount of indapamide into HPMC-ethanol aqueous solution, stir well to a lump-free suspension.
[0054] 2. Granulation: Put the weighed cornstarch, lactose, and povidone K90 into the fluidized bed in sequence, and use top spray granulation. The parameters set in the fluidized bed at different times are shown in the table below:
[0055]
[0056] After spraying the granulation liquid on the top, it is dried, and the drying parameters are set: the material temperature is 45°C, the air inlet temperature is 60°C, and the air volume of the fan is 900m 3 / h. Moisture is controlled below 3.0%.
[0057] 3. Total mixing: Put the 40-mesh sieved granules and the prescribed amount of silicon dioxid...
Embodiment 3
[0059] Example 3 Preparation of Indapamide Capsules (Specification: 2.5mg)
[0060]
[0061] 1. Material preparation: Weigh the raw and auxiliary materials with the required weight and set aside.
[0062] Preparation of granulation solution: Dissolve the prescribed amount of hypromellose E5 in ethanol aqueous solution, make HPMC-ethanol aqueous solution, stir to dissolve, then put the prescribed amount of indapamide into HPMC-ethanol aqueous solution, stir well to a lump-free suspension.
[0063] 2. Granulation: Put the weighed cornstarch, lactose, and povidone K90 into the fluidized bed in sequence, and use top spray granulation. The parameters set in the fluidized bed at different times are shown in the table below:
[0064]
[0065] After spraying the granulation liquid on the top, it is dried, and the drying parameters are set: the material temperature is 50°C, the air inlet temperature is 65°C, and the air volume of the fan is 1200m 3 / h. Moisture is controlled b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com