Synthetic process of fluazinam
A synthesis process, the technology of fluazinam, applied in the field of synthesis process of fluazinam, can solve the problems of high post-treatment purification pressure, unfavorable industrial production, and unit consumption cost of nitrates, so as to save purification steps and reduce nitrates Unit consumption, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
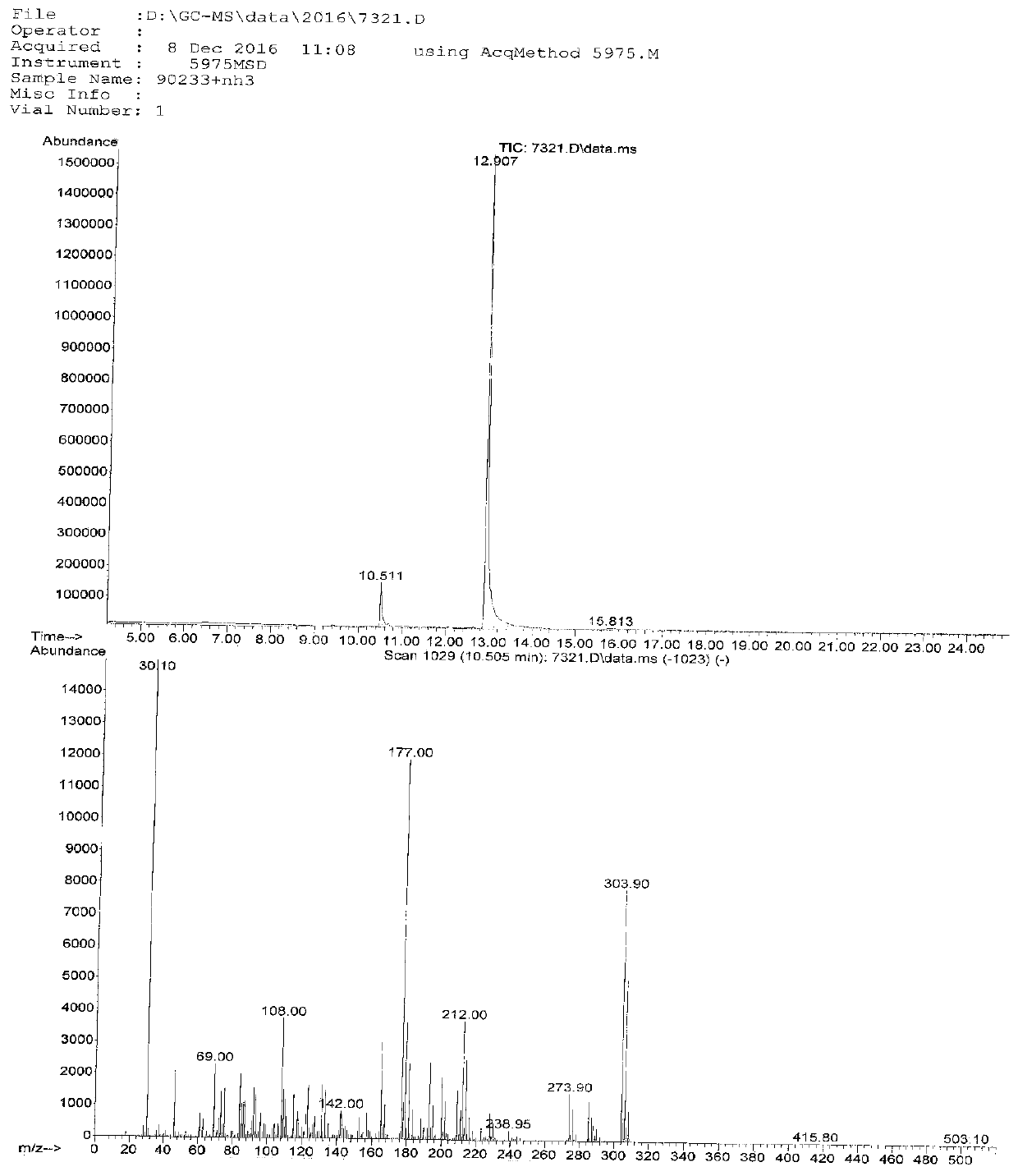
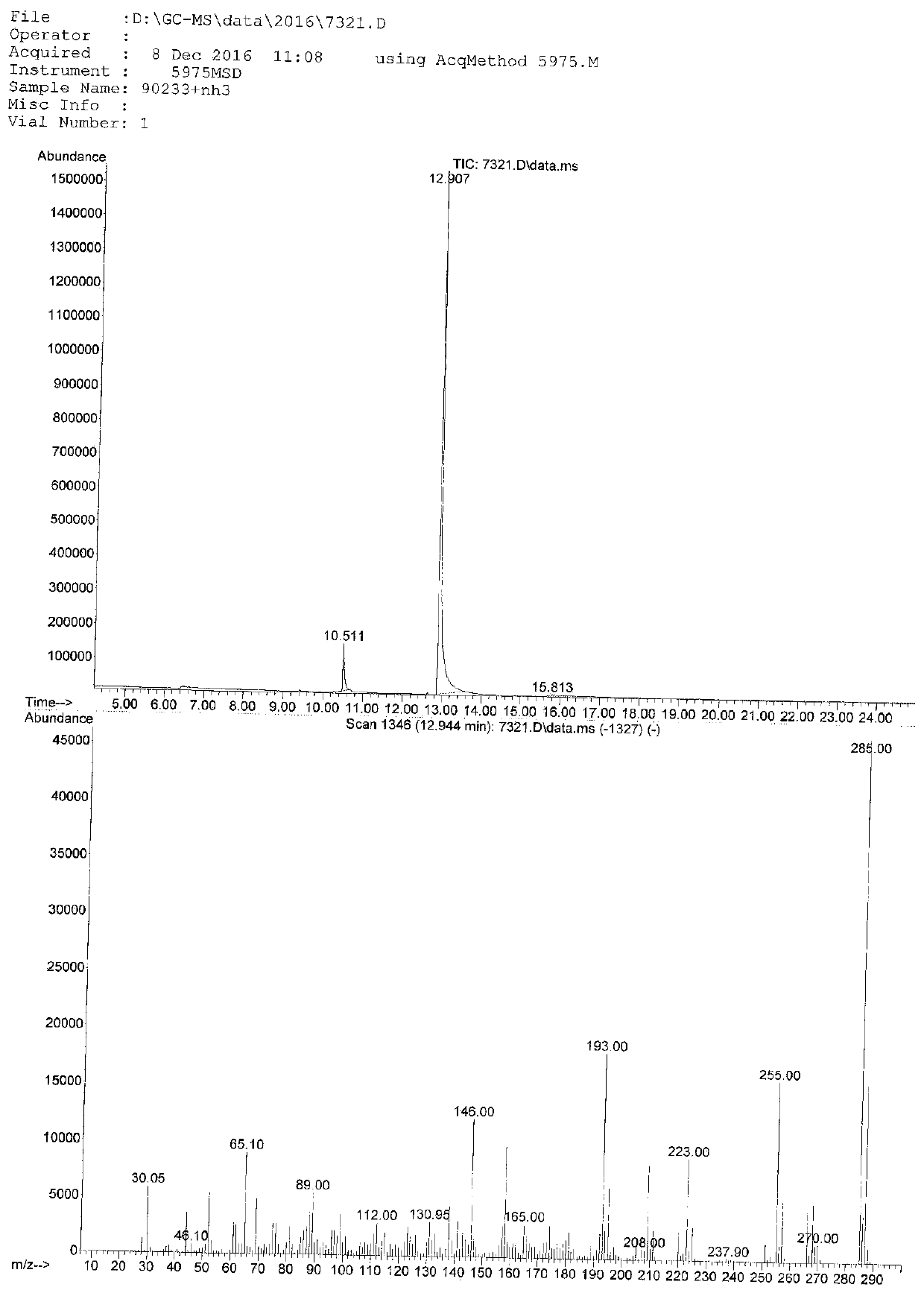
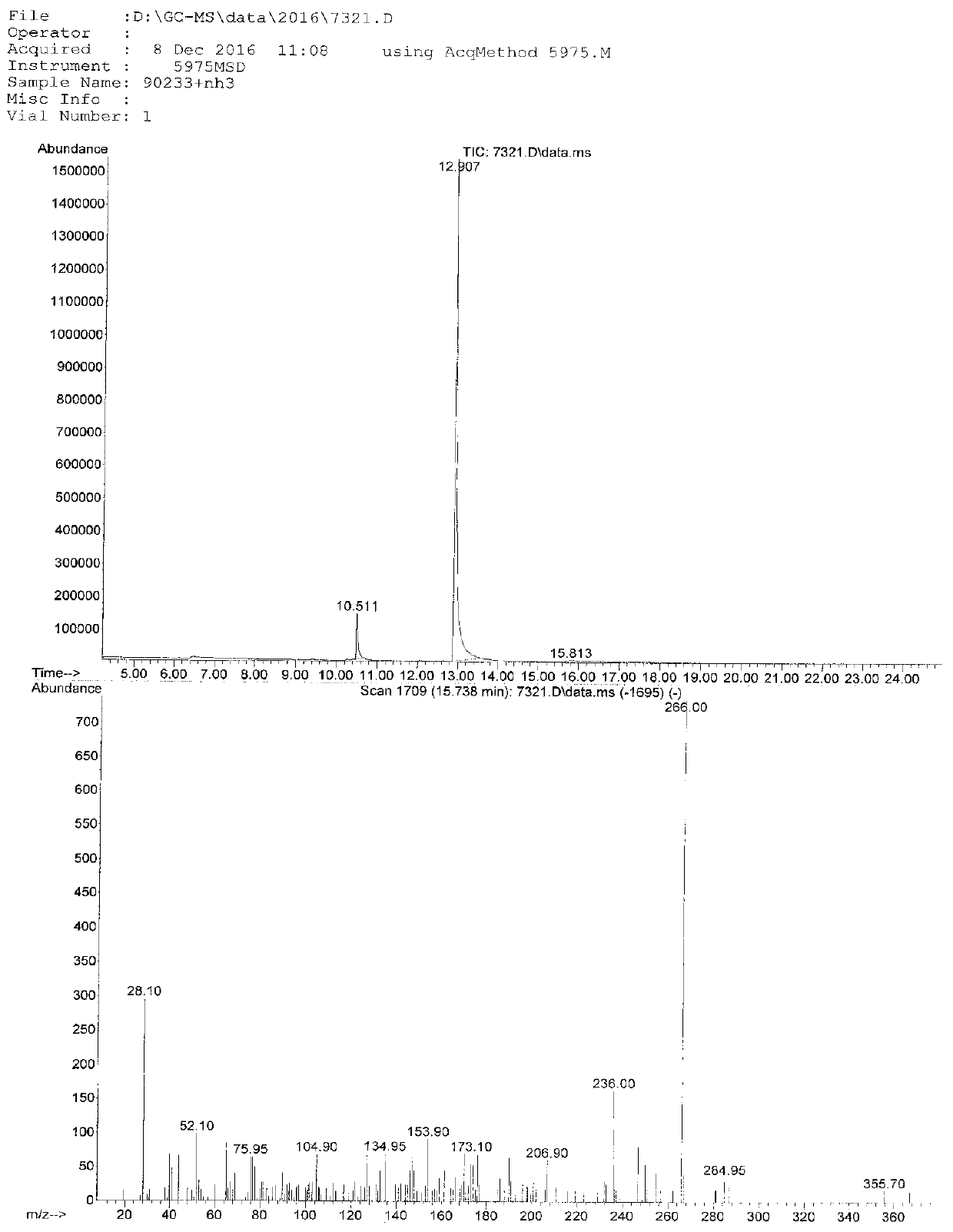
Image
Examples
Embodiment 1
[0026] 51.2 g (0.2348 mol, 99%, 1.0 eq) of 2,3-dichloro-5-trifluoromethylpyridine and 160 mL of tetrahydrofuran were put into a 500 mL autoclave, and the autoclave was closed. Nitrogen was replaced three times at room temperature, 20.0 g (1.174 mol, 5.0 eq) of liquid ammonia was passed into the system, and the internal pressure of the system was about 0.6 Mpa. Raise the temperature to 100°C and hold the pressure for 28 hours. The pressure of the system drops from 1.6Mpa to 1.2Mpa. The reaction of the raw materials controlled by HPLC is completed by inserting a bottom tube to sample. After the system was lowered to normal temperature, the pressure was released to normal pressure, and the released ammonia gas was absorbed with tetrahydrofuran. The reaction solution was taken out, filtered, and rinsed with an appropriate amount of tetrahydrofuran, and the mother liquor was subjected to deamination treatment in the next step.
[0027] The above mother liquor was transferred into ...
Embodiment 2
[0033]47.3 g (0.2348 mol, 99%, 1.0 eq) of 2-fluoro-3-chloro-5-trifluoromethylpyridine and 120 mL of THF were put into a 500 mL autoclave, and the autoclave was closed. Nitrogen was replaced three times, 12.0 g (0.7044 mol, 3.0 eq) of liquid ammonia was passed into the system, and the pressure in the system was about 0.5 Mpa. Raise the temperature to 30°C and hold the pressure for 25 hours. The pressure of the system drops from 0.9 Mpa to 0.7 Mpa. The reaction of the raw materials controlled by HPLC is completed by inserting a bottom tube for sampling. After the system was lowered to normal temperature, the pressure was released to normal pressure, and the released ammonia gas was absorbed with tetrahydrofuran. The reaction solution was taken out, filtered, and the mother liquor was subjected to the next step of deamination treatment.
[0034] Transfer the above mother liquor into a 1000mL four-necked bottle, and remove the ammonia gas under reduced pressure at 10-15°C for 2 h...
Embodiment 3
[0040] 51.2 g (0.2348 mol, 99%, 1.0 eq) of 2,3-dichloro-5-trifluoromethylpyridine and 200 mL of tetrahydrofuran were put into a 500 mL autoclave, and the autoclave was closed. Nitrogen was replaced three times, 40.0 g (2.348 mol, 10.0 eq) of liquid ammonia was passed into the system, and the pressure in the system was about 0.7 Mpa. Raise the temperature to 70°C and hold the pressure for 32 hours. The pressure of the system drops from 1.6 Mpa to 1.2 Mpa, and the reaction of the raw materials controlled in the sampling process is complete. After the system was lowered to normal temperature, the pressure was released to normal pressure, and the released ammonia gas was absorbed with tetrahydrofuran. The reaction solution was taken out, filtered, and the mother liquor was subjected to the next step of deamination treatment.
[0041] The mother liquor was transferred into a 1000 mL four-necked bottle, cooled to -10°C in an ice-salt bath for 3 hours to remove ammonia under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com