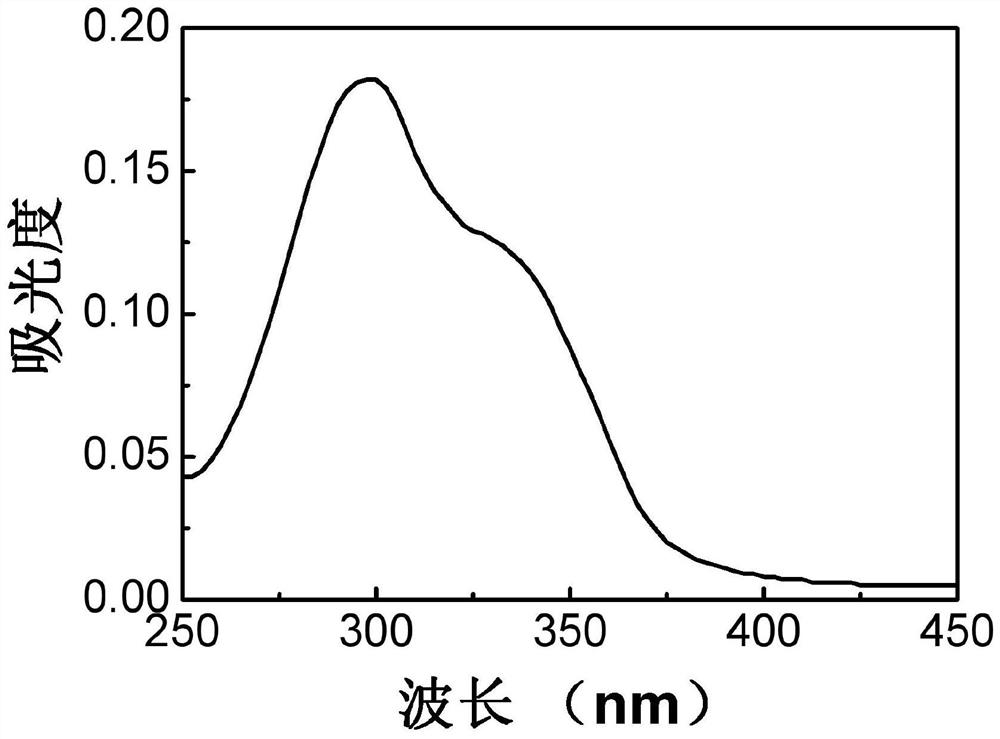
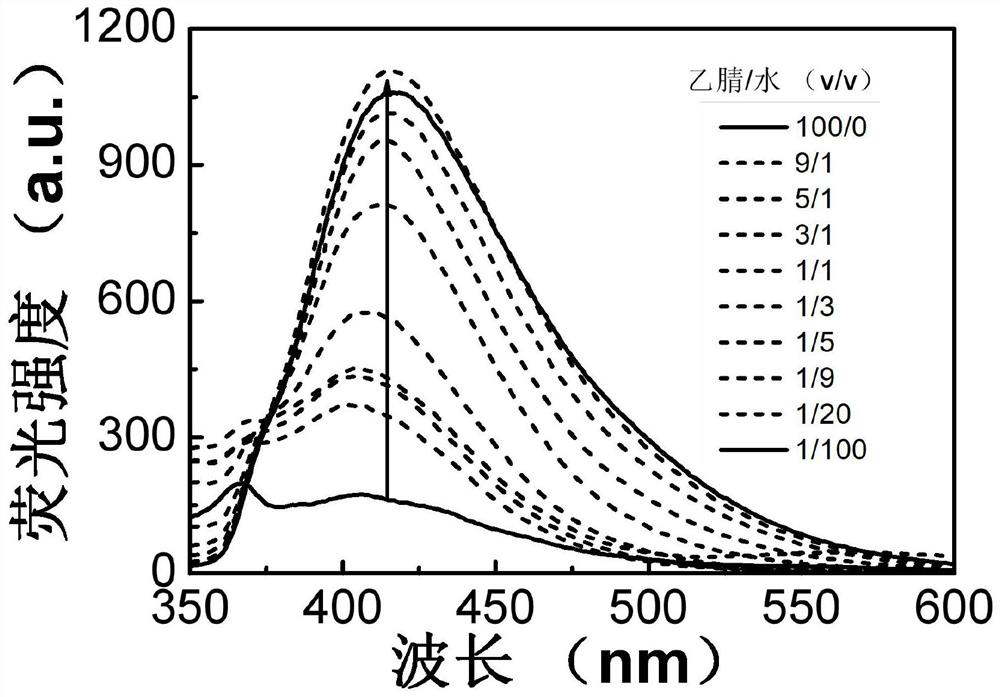
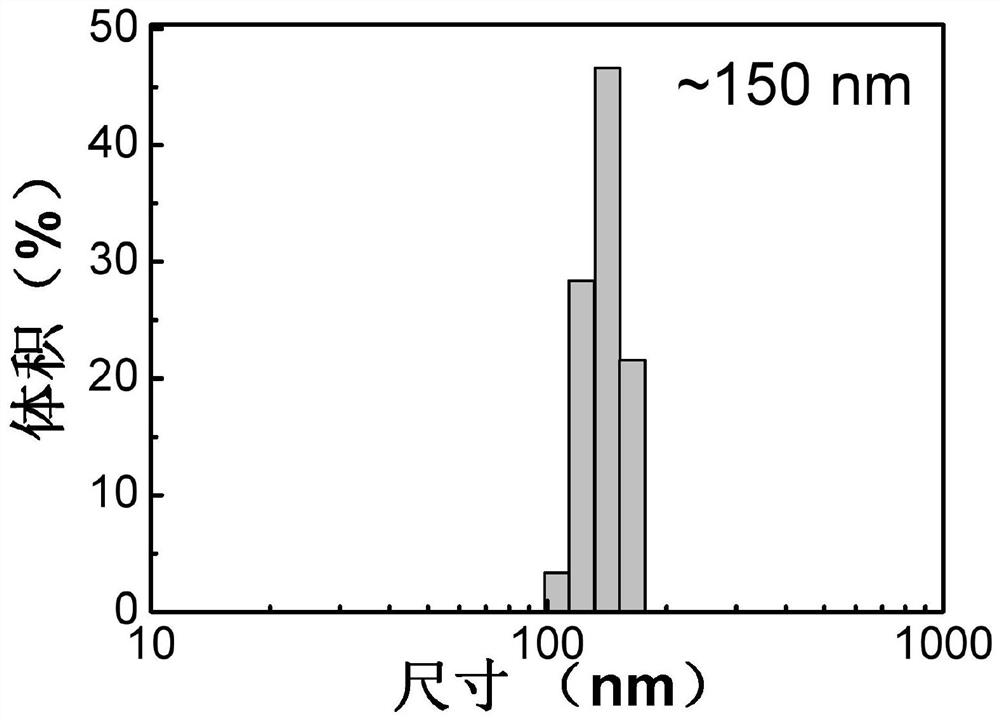
Aggregation-induced luminescent material containing quinoline and coumarin functional groups and preparation method thereof
A technology of aggregation-induced luminescence, coumarin, applied in luminescent materials, chemical instruments and methods, material analysis by optical means, etc. mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The specific preparation method of 2-oxo-N'-(2-(quinolin-8-yloxy)acetyl)-2H-benzopyran-3-carboxhydrazide comprises the following steps:
[0038] 1), prepare 2-(quinolin-8-yloxy)ethyl acetate, refer to the literature (Both visual and ratiometricfluorescent sensor for Zn 2+ based on spirobenzopyran platform, Tetrahedron Letters 53 (2012) 2001-2004.) Preparation;
[0039] The reaction equation is:
[0040]
[0041] 2), preparation of 2-(quinolin-8-yloxy)acetylhydrazide, refer to the literature (Both visual and ratiometricfluorescent sensor for Zn 2+ based on spirobenzopyran platform, Tetrahedron Letters 53 (2012) 2001-2004.) Preparation;
[0042] The reaction equation is:
[0043]
[0044] 3), preparation of 2-oxo-N'-(2-(quinolin-8-yloxy)acetyl)-2H-benzopyran-3-carboxhydrazide,
[0045] Dissolve coumarin-3-carboxylic acid, N-hydroxysuccinimide and dicyclohexylcarbodiimide in DMF, react at room temperature for 8h-10h, filter the product with suction, collect the f...
Embodiment 1
[0048]1), add 0.51g 8-hydroxyquinoline (3.5mmol), 0.48g potassium carbonate (3.5mmol) and 10mL acetone in the 25mL there-necked flask that stirring magnet is housed, reflux and stir for 30 minutes; Then 0.83g bromoacetic acid Ethyl ester (5.0mmol) was added to the stirred solution at one time, and then the reaction mixture was refluxed and stirred for 6h; after the reaction was completed, the solvent was spin-off, extracted with ethyl acetate and saturated brine, the combined organic layers were washed with anhydrous magnesium sulfate After drying and evaporation under reduced pressure, the crude product was obtained. Subsequent separation by column chromatography, select ethyl acetate:petroleum ether (1:3) as eluent, obtain 0.65g yellow oily matter 2-(quinolin-8-yloxy)ethyl acetate (2.8mmol), produce Rate 80%.
[0049] 2), 0.23g of 2-(quinolin-8-yloxy)ethyl acetate (1.0mmol) was dissolved in 5mL of methanol, and 0.51g of hydrazine hydrate (10mmol) was added to react at room ...
Embodiment 2
[0052] 1), add 0.51g 8-hydroxyquinoline (3.5mmol), 0.48g potassium carbonate (3.5mmol), 0.83g ethyl bromoacetate (5.0mmol) and 10mL in a 25mL three-necked flask equipped with a stirring magnet. Acetone, and then the reaction mixture was refluxed and stirred for 6 h; after the reaction was completed, the solvent was removed, extracted with ethyl acetate and saturated brine, the organic layers were combined, dried with anhydrous magnesium sulfate, and evaporated under reduced pressure to obtain a crude product, which was separated by column chromatography. Ethyl acetate:petroleum ether (1:3) was used as eluent to obtain 0.51 g of yellow oily ethyl 2-(quinolin-8-yloxy)acetate (2.2 mmol), with a yield of 62%.
[0053] 2), with embodiment 1.
[0054] 3), 0.19g coumarin-3-carboxylic acid (1.0mmol), 0.14g N-hydroxysuccinimide (1.2mmol) and 0.25g dicyclohexylcarbodiimide (1.2mmol) were dissolved in 5mL DMF , react at room temperature for 10 h, filter the product with suction, collect...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com