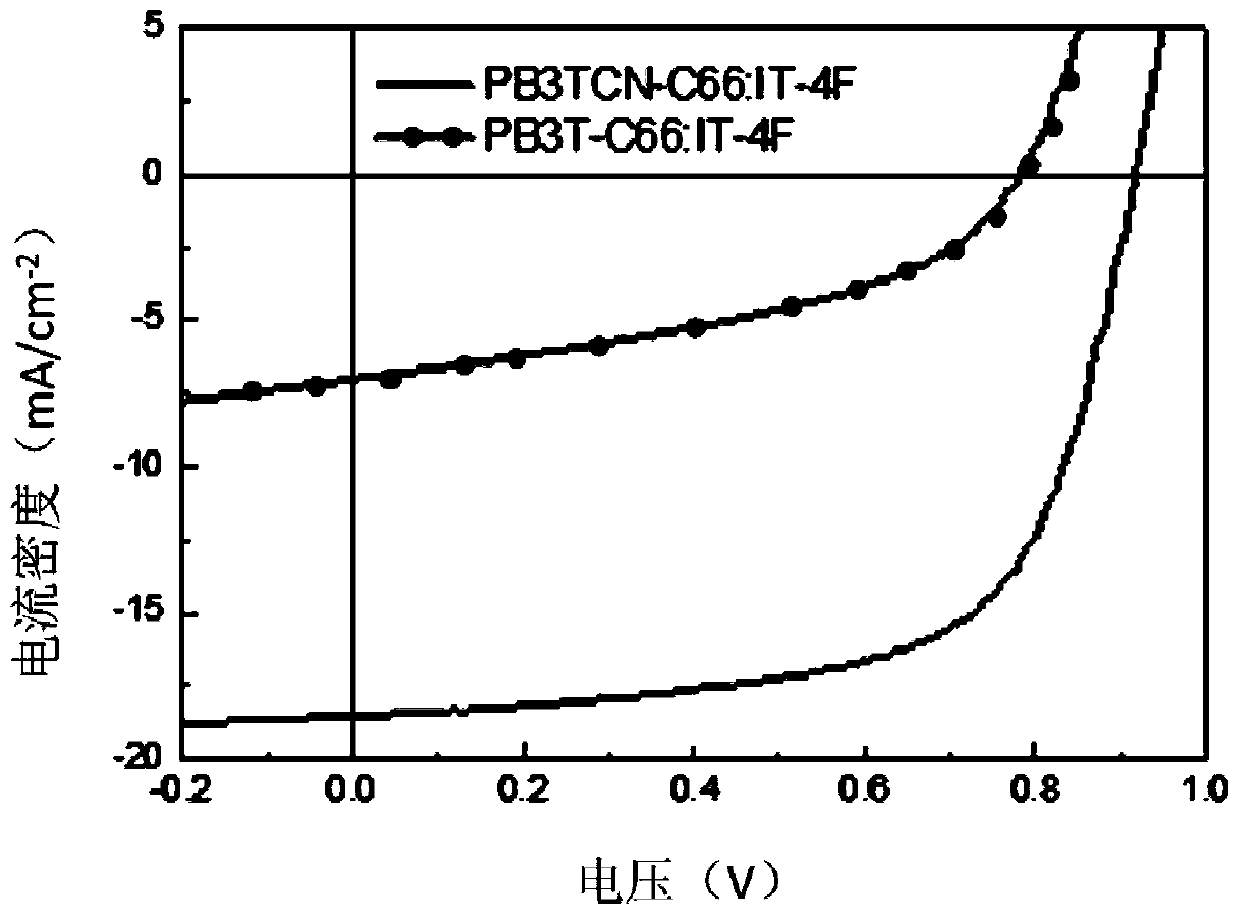
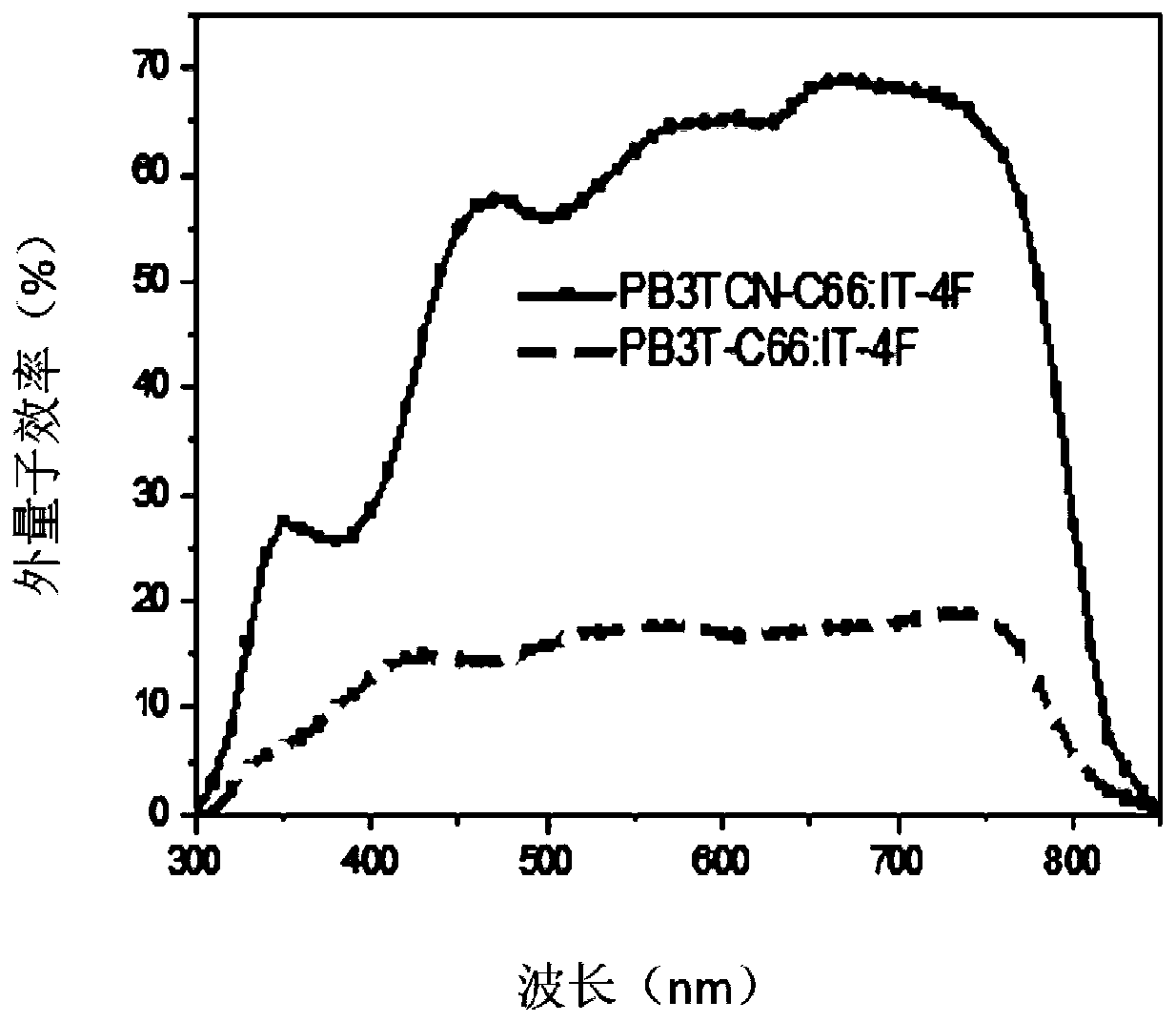
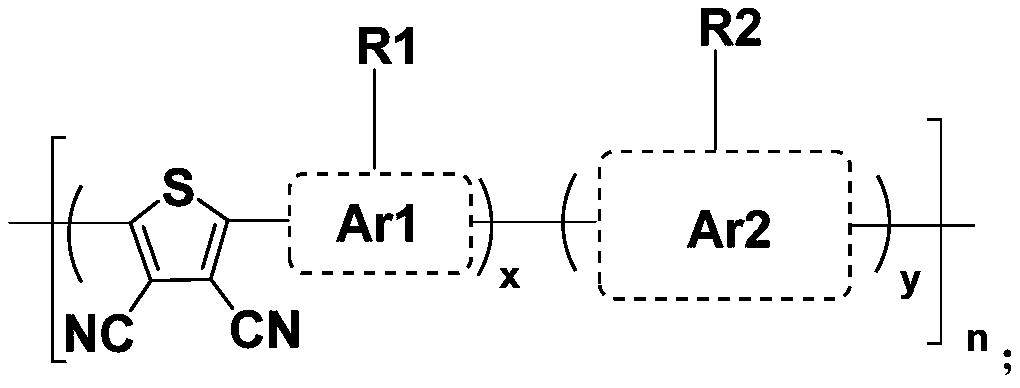
P-type conjugated polymer containing 3,4-dicyanothiophene, and preparation method and application thereof
A conjugated polymer and dicyano technology, applied in the field of p-type conjugated polymers and their preparation, can solve problems such as improvement, and achieve the effects of simple process, high dielectric constant and low manufacturing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Polymer PB3TCN-C66.
[0037] The chemical synthesis route of PB3TCN-C66 is shown below, and the specific reaction steps and reaction conditions are as follows:
[0038]
[0039] (1) Preparation of raw materials or intermediate reactants: raw material 1 according to the method reported in the literature (Wang, Y.; Hasegawa, T.; Matsumoto, H.; Mori, T.; Michinobu, T. Rational Design of High-Mobility Semicrystalline Conjugated Polymers with Tunable Charge Polarity:BeyondBenzobisthiadiazole-Based Polymers, Adv.Funct.Mater.2017,27,1604608.); raw material 2 according to the method reported in the literature (Balandier, J.; Quist, F.; Amato, C.; Bouzakraoui, S.; Cornol, J.; Sergeyev, S.; Geerts, Y. Synthesis of soluble oligothiophenes bearing cyanogroups, their optical and electrochemical properties, Tetrahedron 2010, 66, 9560-9572.) prepared. Raw material 5, tetrahexatriphenylphosphine palladium (Pd(PPh 3 ) 4 ), N-bromosuccinimide (NBS), tris(dibenzyliden...
Embodiment 2
[0047] Preparation of polymer PB3TCN-EH-EH.
[0048] The chemical synthesis route of PB3TCN-EH-EH is shown below, and the specific reaction steps and reaction conditions are as follows:
[0049]
[0050] (1) Preparation of raw materials or intermediate reactants: raw material 6 according to the method reported in the literature (Bundgaard, E.; Krebs, F.Low-Band-Gap Conjugated Polymers Based on Thiophene, Benzothiadiazole, and Benzobis (thiadiazole) Macromolecules 2006,39, 2823-2831.) According to the method reported in the literature (Balandier, J.; Quist, F.; Amato, C.; Bouzakraoui, S.; Cornol, J.; Sergeyev, S.; Geerts, Y.Synthesis of soluble oligothiophenes bearing cyano groups, theiroptical and electrochemical properties, Tetrahedron 2010, 66, 9560-9572.) prepared. Raw material 10, tetrahexa triphenylphosphine palladium (Pd(PPh 3 ) 4 ), N-bromosuccinimide (NBS), tris(dibenzylideneacetone) dipalladium (Pd 2 (dba) 3 ), three (o-methylphenyl) phosphorus (P (o-tol) 3 )...
Embodiment 3
[0058] Preparation of polymer PB3TCN-EH-C12.
[0059] The chemical synthesis route of PB3TCN-EH-C12 is shown below, and the specific reaction steps and reaction conditions are as follows:
[0060]
[0061] (1) Preparation of raw materials or intermediate reactants: raw material 11 according to the method reported in the literature (Wu, P.; Kim, F.; Champion, R.; Jenekhe, S. Conjugated Donor-Acceptor Copolymer Semiconductors. Synthesis, Optical Properties, Electrochemistry , and Field-EffectCarrier Mobility of Pyridopyrazine-Based Copolymers.Macromolecules 2008,41,7021-7028.) Raw material 12 according to the method reported in the literature (Balandier, J.; Quist, F.; Amato, C.; Bouzakraoui, S.; Cornol, J.; Sergeyev, S.; Geerts, Y. Synthesis of soluble oligothiophenes bearing cyano groups, their optical and electrochemical properties, Tetrahedron 2010, 66, 9560-9572.)) prepared. Raw material 15, tetrahydryl triphenylphosphine palladium (Pd(PPh 3 ) 4 ), N-bromosuccinimide ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com