Medicine materials for ketoprofen pasters and preparation method of medicine materials
A technology of ketoprofen and patch, which is applied in the direction of medical formula, non-active ingredient medical preparations, medical preparations containing active ingredients, etc., can solve the problem of less solid loading of active ingredient ketoprofen and poor slow-release effect. Good, short drug effect time, etc., to meet the needs of clinical treatment, good application prospects, and long drug effect time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
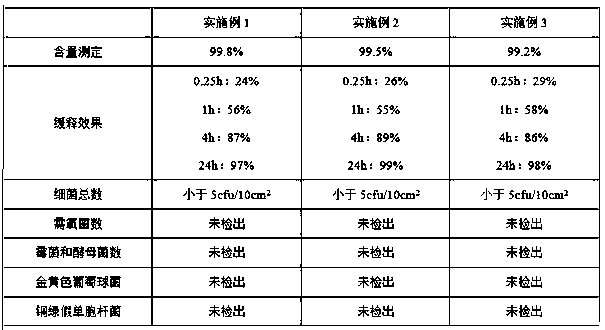
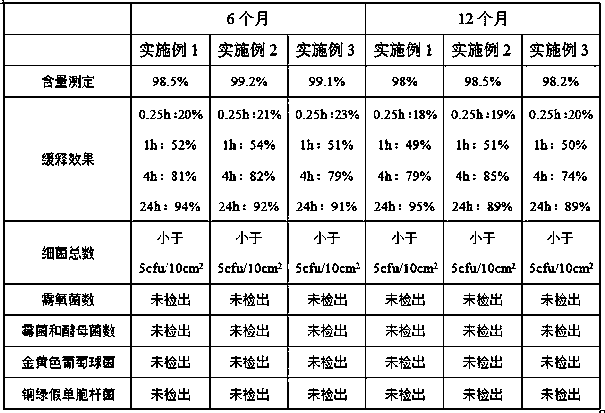
Embodiment 1
[0022] 1) Weigh the following components in parts by weight: 5 parts of ketoprofen, 2 parts of polyethylene glycol, 1 part of polyvinyl alcohol, 2 parts of carboxymethyl chitosan, 2 parts of lauryl azone, 1 part of oleic acid 20 parts and hot melt adhesive;
[0023] 2) Stir and dissolve carboxymethyl chitosan, polyethylene glycol, polyvinyl alcohol and oleic acid evenly, then slowly add ketoprofen and laurocaprazine, stir and dissolve evenly to obtain a mixed solution, and then mix the The mixed solution was placed at 110°C, reacted for 2.0h, and stirred at 8000rpm for 60min to make it evenly mixed until no particles were visible to the naked eye. After cooling to room temperature, it was sterilized by filtration with a 0.22μm ultrafiltration membrane to obtain solution I;
[0024] 3) Put the hot melt adhesive into the melter, heat it to 120°C for 2.0 hours, and melt the glue block into a milky white paste;
[0025] 4) Transfer the melted hot melt adhesive in step 3) from the...
Embodiment 2
[0027] 1) Weigh the following components in parts by weight: 8 parts of ketoprofen, 4 parts of polyethylene glycol, 1 part of polyvinyl alcohol, 3 parts of carboxymethyl chitosan, 3 parts of lauryl azone, 2 parts of oleic acid 23 parts and hot melt adhesive;
[0028] 2) Stir and dissolve carboxymethyl chitosan, polyethylene glycol, polyvinyl alcohol and oleic acid evenly, then slowly add ketoprofen and laurocaprazine, stir and dissolve evenly to obtain a mixed solution, and then mix the The mixed solution was placed at 120°C, reacted for 1.5h, and stirred at 10,000rpm for 30min to make it evenly mixed until no particles were visible to the naked eye. After cooling to room temperature, filter and sterilize with a 0.22μm ultrafiltration membrane to obtain solution I;
[0029] 3) Put the hot melt adhesive into the melter, heat it to 125°C for 2.0 hours, and melt the glue block into a milky white paste;
[0030] 4) Transfer the melted hot melt adhesive in step 3) from the melt ma...
Embodiment 3
[0032] 1) Weigh the following components in parts by weight: 10 parts of ketoprofen, 6 parts of polyethylene glycol, 4 parts of carboxymethyl chitosan, 1 part of polyvinyl alcohol, 4 parts of laurocaprine, 2 parts of oleic acid 24 parts and hot melt adhesive;
[0033] 2) Stir and dissolve carboxymethyl chitosan, polyethylene glycol, polyvinyl alcohol and oleic acid evenly, then slowly add ketoprofen and laurocaprazine, stir and dissolve evenly to obtain a mixed solution, and then mix the The mixed solution was placed at 130°C, reacted for 1.5h, and stirred at 9000rpm for 40min to make it evenly mixed until no particles were visible to the naked eye. After cooling to room temperature, it was sterilized by filtration with a 0.22μm ultrafiltration membrane to obtain solution I;
[0034] 3) Put the hot melt adhesive into the melter, heat it to 140°C for 1.5 hours, and melt the glue block into a milky white paste;
[0035] 4) Transfer the melted hot melt adhesive in step 3) from t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com