Preparation method and application of anti-tumor nano arsenic spheres based on invertebrate recombinant ferritin
A technology of recombining ferritin and invertebrates, applied in the field of preparation of anti-tumor nano arsenic spheres, can solve problems such as toxic side effects, death, and unsatisfactory treatment effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0033] Preparation of recombinant ferritin based on invertebrates
[0034] 1. Cloning of ferritin gene sequence
[0035] 1.1 Gene amplification
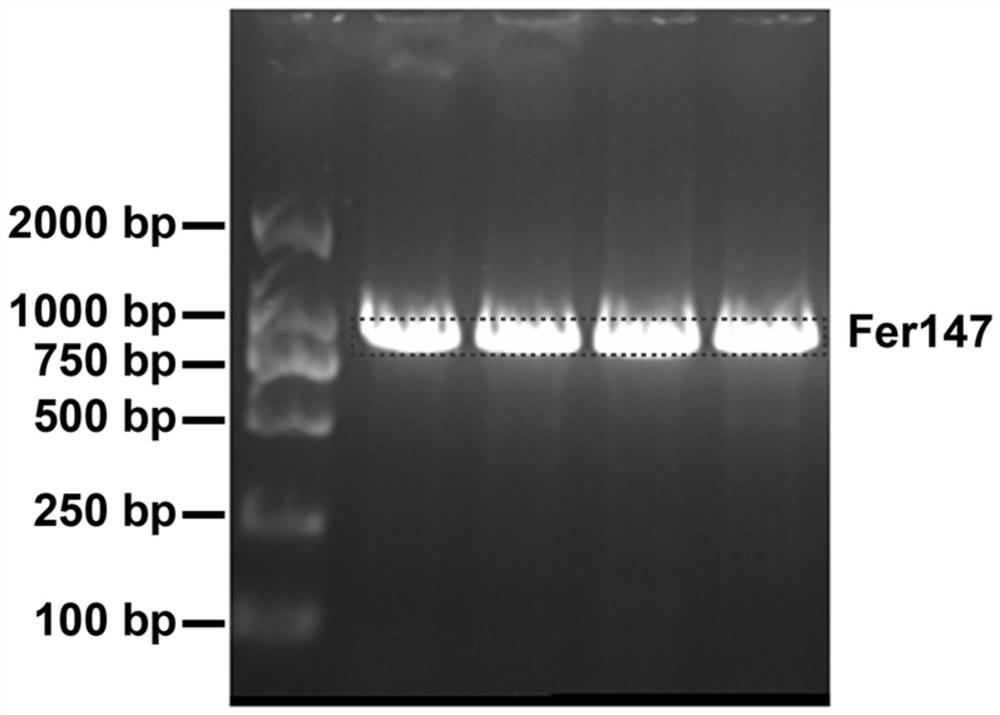
[0036] Using the Fer147 sequence (SUMO+ferritin gene) as a template, use Primer Primer 5.0 software to design the upstream expression primer FER-F: 5′- CCGCTCGAGAATTAGGAGGAAGTCCAAGA-3′; and the downstream expression primer FER-R: 5′- CGCCATATGTCGGACTCAGAAGTCAATCA-3′, PCR The target gene of Fer147 was amplified, and the PCR product was recovered and purified by gel recovery kit, and after confirmation by sequencing comparison, the target gene of Fer147 was obtained. (Fer147 is a new protein—Fer147, which can interact with ferritin of D. pachyrhiza, which was screened out by the research group using the yeast two-hybrid system. Through sequence analysis, it was found that Fer147 is a member of the ferritin family, and its full-length DNA sequence contains 916 bases, composed of 174 amino acids. The gene sequence of Fer147 has not bee...
specific Embodiment 2
[0055] enrichment of heavy metals
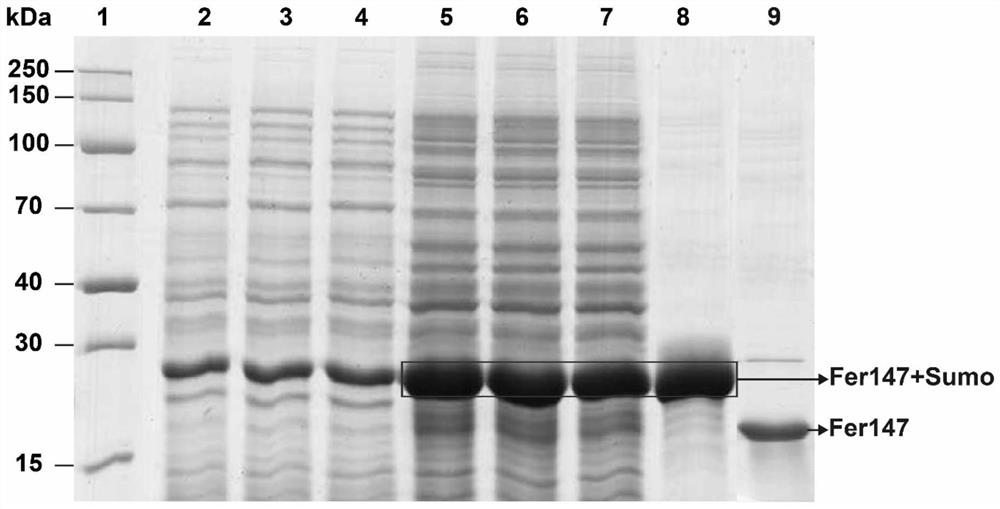
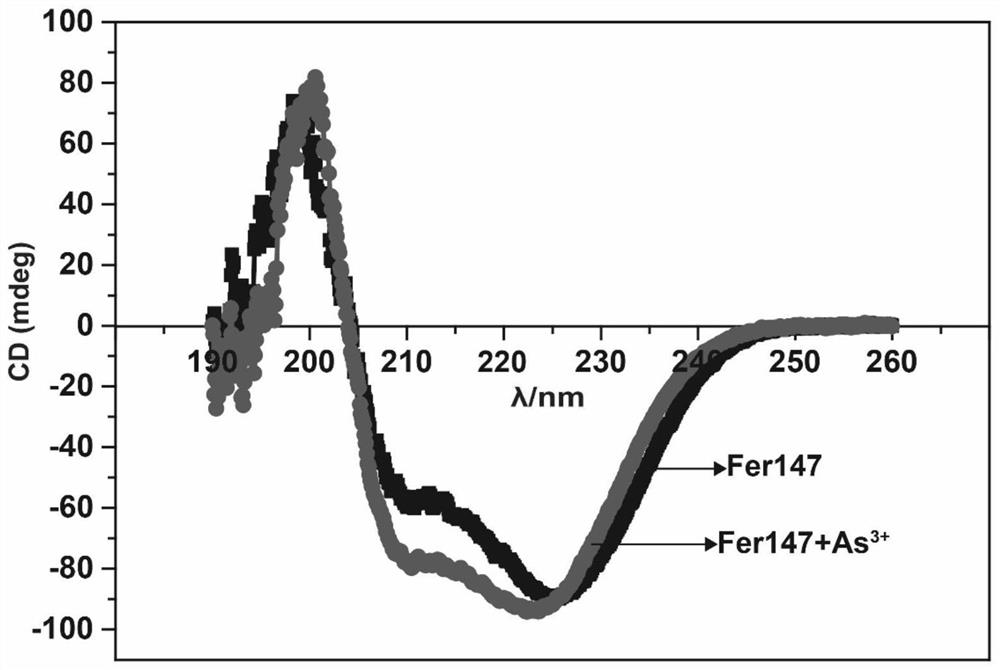
[0056] 1. Based on the pre-experimental results, after determining the concentration range of the metal solution in which ferritin is enriched with heavy metal ions, 5 mM metal solution is selected as the enrichment concentration. Put the purified ferritin without the SUMO enzyme tag into the dialysis bag, in 5 mM sodium arsenite (NaAsO 2 ) solution, and a magnetic stirrer was used to simulate flowing liquid during the entire enrichment process. After 12 hours of dialysis, the dialysis bag was dialyzed in protein buffer (25 mM Tris, 150 mM NaCl, pH 8.0) for 12 hours, during which each The protein buffer was replaced every 4 hours to remove heavy metal ions not adsorbed by the recombinant protein. The protein solution in the dialysis bag was taken out and placed in an ultrafiltration tube. According to the requirements of subsequent experiments, the protein was concentrated and stored at -80 °C for future use.
[0057] 2. Determination of F...
specific Embodiment 3
[0067] Crystal preparation and resolution
[0068] 1. Preparation of protein samples
[0069] (1) Thaw frozen ferritin samples on ice from a -80°C refrigerator (centrifuge directly for fresh samples), centrifuge the thawed protein samples in a high-speed refrigerated centrifuge at 4°C, 12,000 rpm for 15 min, and remove Denatured protein precipitates and impurities, and ensure the uniformity of the sample. Transfer the supernatant solution after centrifugation to a new pre-cooled centrifuge tube. If there are many foams in the solution during the transfer process, centrifuge for 5 minutes to remove the foam. ;
[0070] (2) Determination of protein concentration: Use the BCA protein concentration determination kit to measure the protein concentration, determine the concentration of the remaining protein after centrifugation, and provide a reference for the subsequent determination of the optimal protein crystallization concentration.
[0071] 2. Primary screening of crystalliz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com