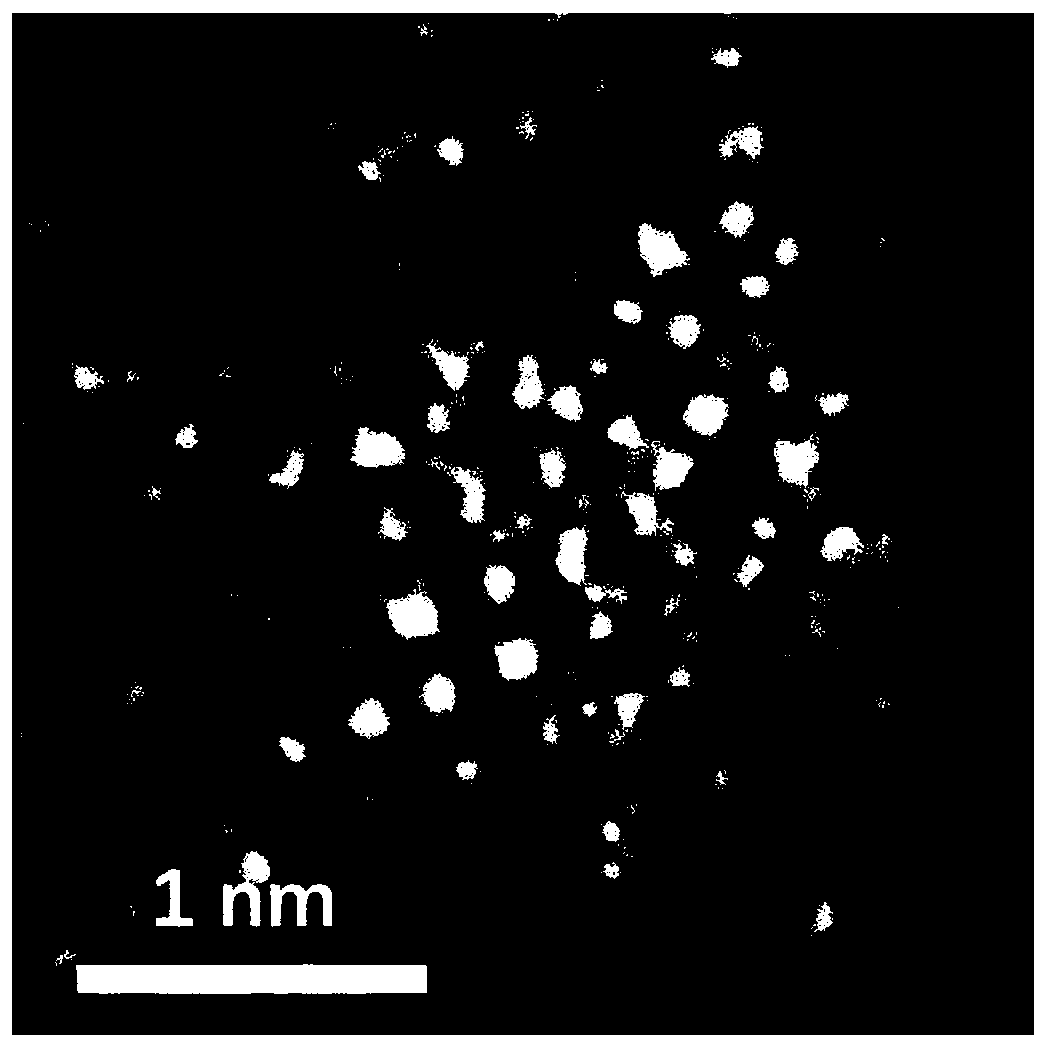
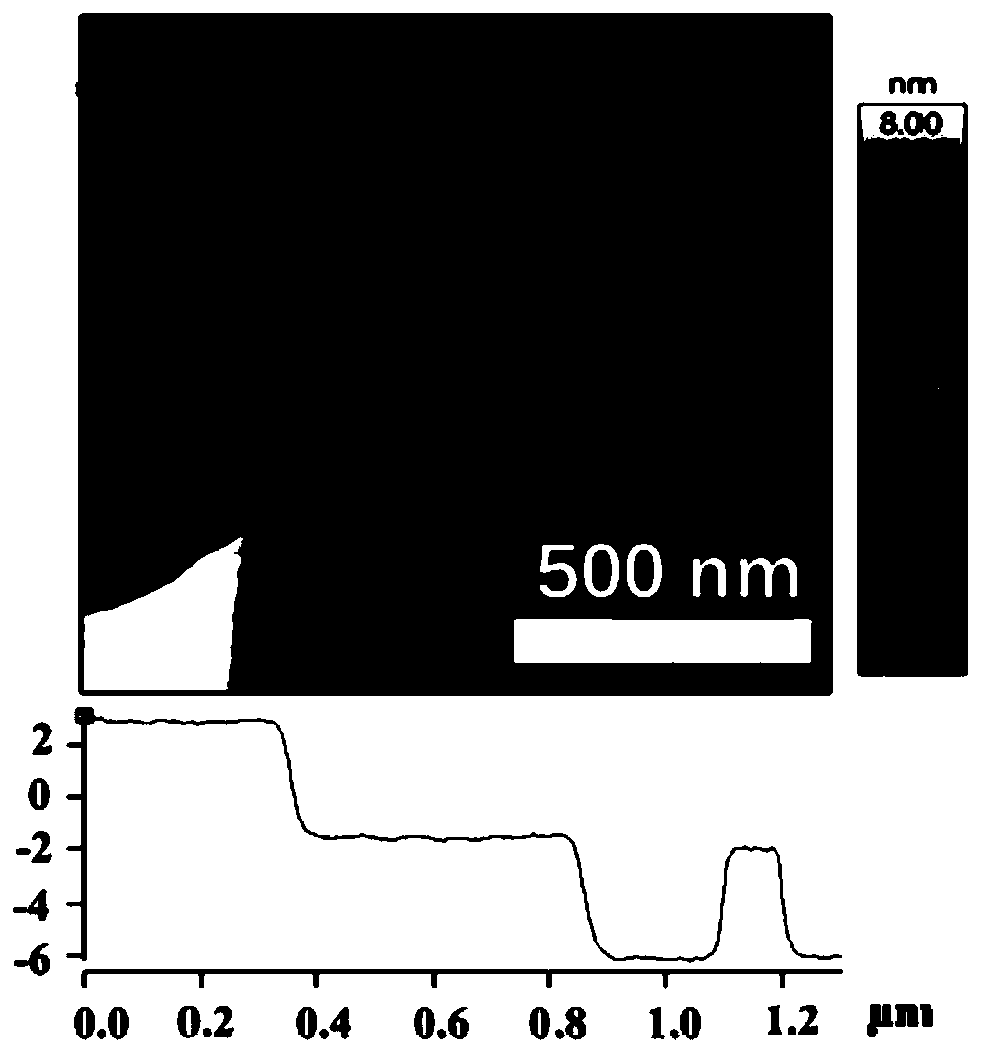
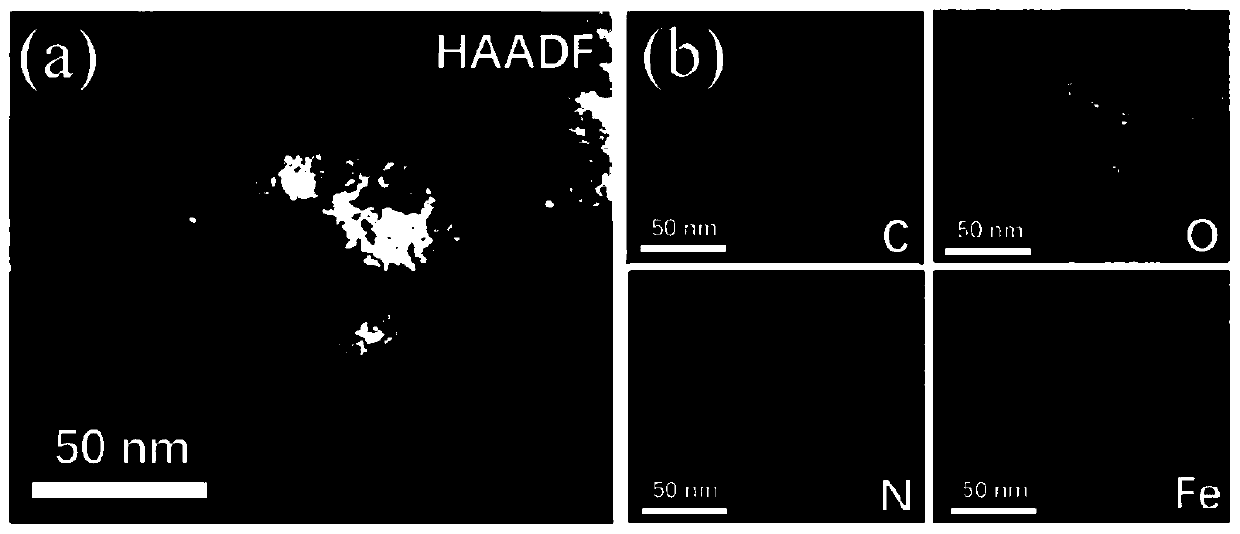
Preparing method of carbon-loaded monoatomic metal nitrogen-containing compound oxygen reduction catalyst and obtained catalyst
A technology of composites and catalysts, applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problem that catalysts are not resistant to acid and alkali electron transport, cannot be prepared on a large scale, and have low pH universality, etc. problems, to achieve the effect of favorable electron transport, excellent catalytic performance, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of Fe-SAC catalyst
[0039] Put 3 g of chitin in a muffle furnace, raise the temperature to 250° C. and keep it for 2 hours to obtain pre-oxidized chitin-based cracked carbon. After cooling to room temperature, the pre-oxidized chitin-based pyrolysis carbon was taken out, and 3 g of ferric chloride solid (as a transition metal source) and 9 g of zinc chloride solid (as a pore-forming agent) were added and mixed uniformly to obtain a reaction mixture. The reaction mixture was put into a porcelain boat (length 80mm*width 60mm*height 30mm), and then placed in a laboratory tube furnace. Nitrogen gas was introduced into the furnace to replace the air, and then the furnace was heated to 800° C. at a temperature increase rate of 5° C. / min, and the reaction mixture was roasted for 2 hours to obtain a roasted product. After cooling to room temperature, pickle with a sufficient amount of hydrochloric acid solution with a concentration of 2 mol / L for...
Embodiment 2
[0040] Embodiment 2: the preparation of Co-SAC catalyst
[0041] Put 3 g of chitin in a muffle furnace, raise the temperature to 200° C. and keep it for 5 hours to obtain pre-oxidized chitin-based cracked carbon. After cooling to room temperature, the pre-oxidized chitin-based pyrolysis carbon was taken out, 12g of cobalt sulfate solid (as a transition metal source) and 6g of zinc chloride (as a pore-forming agent) were added, and mixed uniformly to obtain a reaction mixture. The reaction mixture was placed in a porcelain boat and placed in a laboratory tube furnace. Nitrogen gas was introduced into the furnace to replace the air, and then the furnace was heated to 1100° C. at a rate of 10° C. / min, and the reaction mixture was calcined for 1 hour to obtain a calcined product. After cooling to room temperature, pickle with a sufficient amount of sulfuric acid solution with a concentration of 1mol / L for 40 hours, and then wash with a sufficient amount of deionized water for sev...
Embodiment 3
[0042] Embodiment 3: the preparation of Mn-SAC catalyst
[0043] Put 3 g of chitin in a muffle furnace, raise the temperature to 300° C. and keep for 1 hour to obtain pre-oxidized chitin-based cracked carbon. After cooling to room temperature, the pre-oxidized chitin-based pyrolysis carbon was taken out, and 6 g of manganese nitrate solid (as a transition metal source) and 12 g of zinc chloride solid (as a pore-forming agent) were added and mixed uniformly to obtain a reaction mixture. The reaction mixture was placed in a porcelain boat and placed in a laboratory tube furnace. Nitrogen gas was introduced into the furnace to replace the air, and then the furnace was heated to 1100° C. at a rate of 10° C. / min, and the reaction mixture was calcined for 1 hour to obtain a calcined product. After cooling to room temperature, pickle with a sufficient amount of nitric acid solution with a concentration of 1mol / L for 25 hours, and then wash with a sufficient amount of deionized water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com