Method for biocatalytic synthesis of alkylpyrazine containing monomethyl semi ring
A technology for the synthesis of alkylpyrazines catalyzed by monomethylsemicycloalkylpyrazine and threonine dehydrogenase, which is applied in fermentation and other fields, and can solve problems such as poor selectivity, difficult separation, and lack of biochemical pathways
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
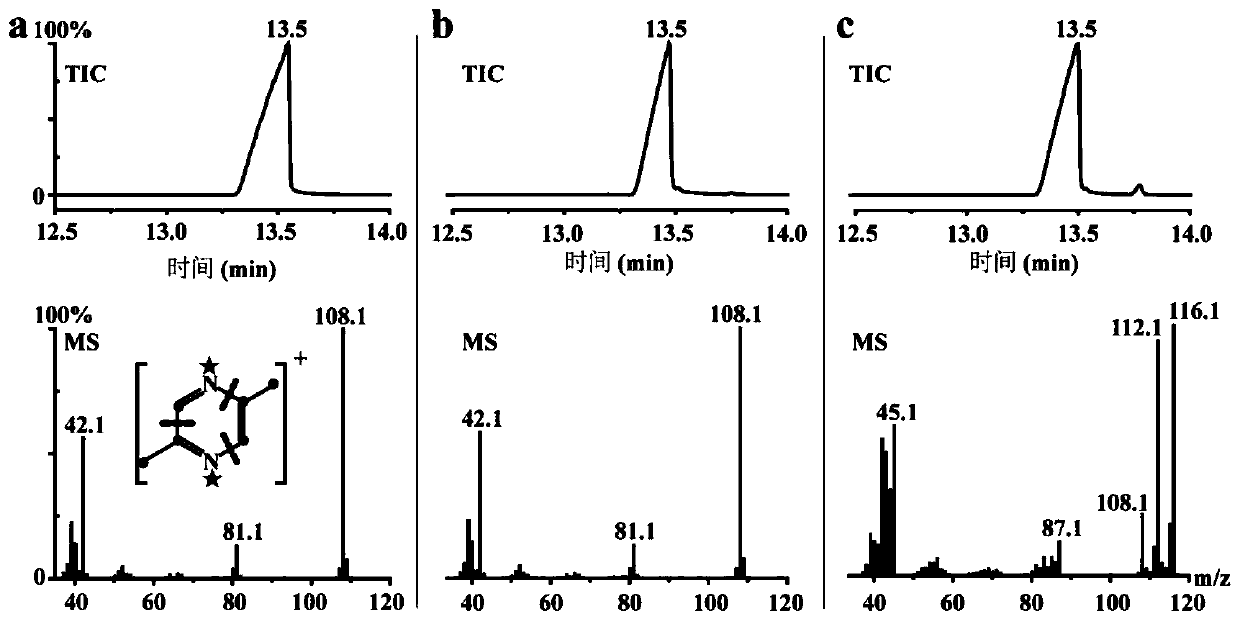
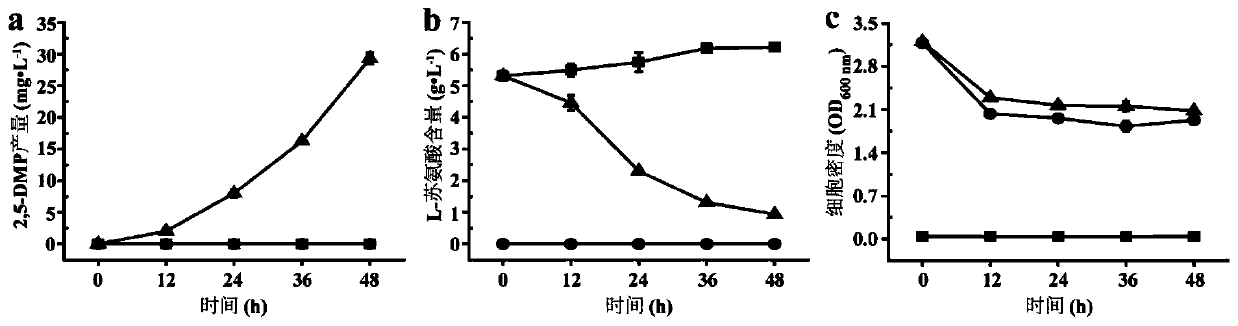
[0071] Example 1: L-threonine can be used as the only substrate source for 2,5-DMP microbial synthesis
[0072] 1. Isotope tracer experiment
[0073] Add 1g·L respectively -1 L-threonine or [U- 13 C, 15 Add N]-L-threonine to LB medium, cultivate B. subtilis 168 to 2d at 37°C and 200rpm, use GC-MS to qualitatively analyze the 2,5-DMP in the fermentation broth, and obtain the total ion pattern ( TIC) and mass spectrometry (MS) such as figure 1 shown. After adding L-threonine and [U- 13 C, 15 In the fermentation sample of N]-L-threonine, it is 13.5min ( figure 1 There are chromatographic peaks appearing at b,c), which is consistent with the retention time of the standard 2,5-DMP ( figure 1 a). Mass spectra of compounds detected in L-threonine spiked samples ( figure 1 b) with 2,5-DMP standard mass spectrum ( figure 1 a) It is completely consistent, indicating that the compound at this peak time in the fermentation sample is 2,5-DMP.
[0074] [U- 13 C, 15 The mass sp...
Embodiment 2
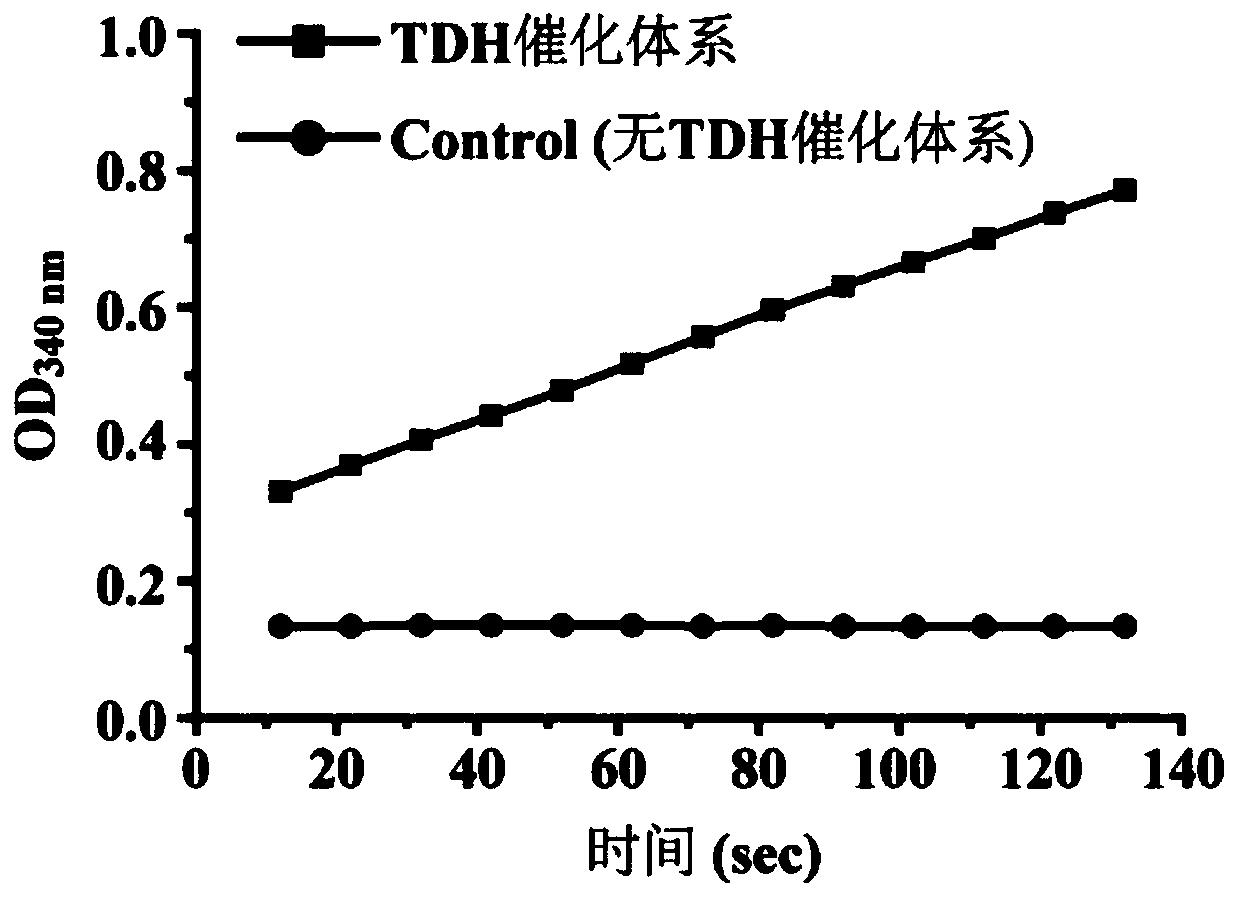
[0079] Example 2: Effect of TDH on the synthesis of 2,5-DMP by microorganisms using L-threonine
[0080] TDH (NP_389581) derived from B. subtilis 168 can convert L-threonine to 2,5-DMP in vitro.
[0081] 1. Exogenous expression and purification of TDH
[0082] Construct the recombinant plasmid pET-28a-tdh as follows:
[0083] Using B. subtilis 168 genomic DNA as a template, the primer tdh-F / R was used to amplify the tdh gene by PCR to obtain the gene fragment tdh. The purified gene fragment tdh and plasmid pET-28a were subjected to double digestion (BamHI / HindIII) respectively, and the digested products were column purified. The digested gene fragment tdh was ligated with plasmid pET-28a, and transformed into E.coli BL21(DE3) competent cells. The recombinant plasmids were extracted from the transformants, and the extracted plasmids were verified by double enzyme digestion (BamHI / HindIII), and the positive clones were selected and sent to Tianlin Biotechnology (Wuxi) Co., Lt...
Embodiment 3
[0110] Example 3: TDH is involved in the microbial synthesis of trimethylpyrazine (TMP)
[0111] 1. Isotope tracer verification of the substrate source of TMP microbial synthesis
[0112] Whether TMP can be derived from L-threonine and D-glucose was verified by using the method of isotope tracing. 4 + Add [U- 13 C 4 , 15 N]-L-threonine and [U- 13 C 6 ]-D-glucose, L-threonine and [U- 13 C 6 ]-D-glucose, [U- 13 C 4 , 15 N]-L-threonine and D-glucose, L-threonine and D-glucose, cultured B.subtilis 168 (cultivation conditions: B.subtilis 168 was activated to obtain seed liquid, and transferred to 1% inoculum Connect to 2mL and add [U- 13 C 4 , 15 N]-L-threonine (1g L -1 ) and [U- 13 C 6 ]-D-glucose (2g·L -1 ), [U- 13 C 4 , 15 N]-L-threonine (1g L -1 ) and D-glucose (2g·L -1 ), L-threonine (1g L -1 ) and [U- 13 C 6 ]-D-glucose (2g·L -1), L-threonine (1g L -1 ) and D-glucose (2g·L -1 ) containing 3g·L -1 Diammonium hydrogen phosphate in LB test tubes, cu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com