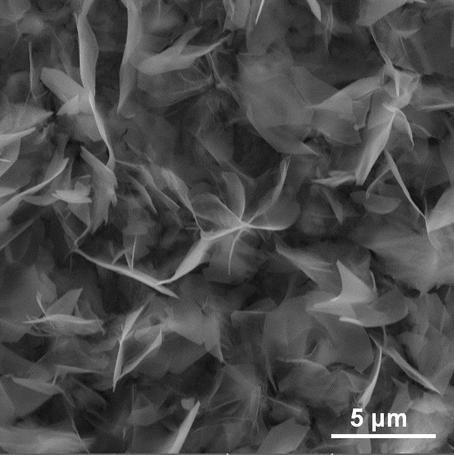
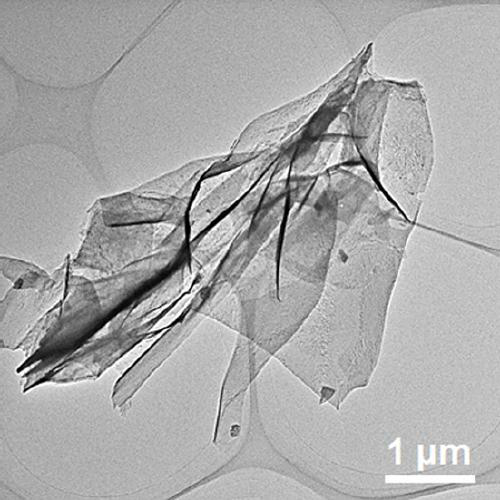
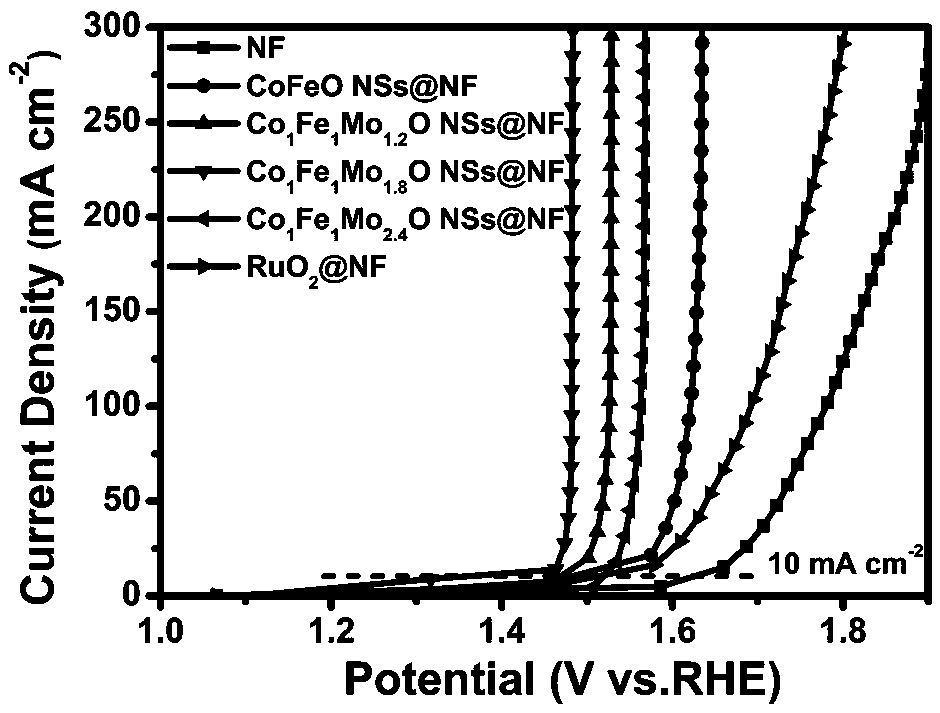
Preparation method of molybdenum-doped cobalt-iron oxide nano-sheet bifunctional electrocatalyst
A technology of cobalt iron oxide and electrocatalyst, which is applied in the field of two-dimensional electrocatalyst preparation, can solve the problems of restricting large-scale application and high cost, and achieve excellent OER activity and stability, easy operation, and excellent long-term cycle durability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] co 1 Fe 1 Mo 1.2 Preparation of O NSs
[0027] First, 1 mmol cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O), 1 mmol ferric nitrate nonahydrate (Fe(NO 3 ) 2 9H 2 O), 5 mmol urea and 4 mmol NH 4 F, and 1.2 mmol of ammonium molybdate tetrahydrate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) added to 36 mL of ultrapure water, stirred until completely dissolved, in which Co(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 2 9H 2 O and (NH 4 ) 6 Mo 7 o 24 4H 2 The O molar x / y / z ratio is 1:1:1.2.
[0028] Then, this solution and the nickel foam as the substrate material were transferred into a 50 mL polytetrafluoroethylene-lined stainless steel autoclave, which was sealed and heated at 180 °C for 10 h, and then naturally cooled to room temperature. The product was washed several times with ultrapure water and absolute ethanol, dried under vacuum at 60°C, and finally annealed at 800°C for 2 hours. The resulting product is denoted as Co 1 Fe 1 Mo 1.2 O NSs@NF.
Embodiment 2
[0030] co 1 Fe 1 Mo 1.8 Preparation of O NSs
[0031] First, 1 mmol cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O), 1 mmol ferric nitrate nonahydrate (Fe(NO 3 ) 2 9H 2 O), 5 mmol urea and 4 mmol NH 4 F, and 1.8 mmol of ammonium molybdate tetrahydrate ((NH 4 )6 Mo 7 o 24 4H 2 O) added to 36 mL of ultrapure water, stirred until completely dissolved, in which Co(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 2 9H 2 O and (NH 4 ) 6 Mo 7 o 24 4H 2 The O molar x / y / z ratio is 1:1:1.8.
[0032] Then, this solution and the nickel foam as the substrate material were transferred into a 50 mL polytetrafluoroethylene-lined stainless steel autoclave, which was sealed and heated at 180 °C for 10 h, and then naturally cooled to room temperature. The product was washed several times with ultrapure water and absolute ethanol, dried under vacuum at 60°C, and finally annealed at 800°C for 2 hours. The resulting product is denoted as Co 1 Fe 1 Mo 1.8 O NSs@NF.
Embodiment 3
[0034] co 1 Fe 1 Mo 2.4 Preparation of O NSs
[0035] First, 1 mmol cobalt nitrate hexahydrate (Co(NO 3 ) 2 ·6H 2 O), 1 mmol ferric nitrate nonahydrate (Fe(NO 3 ) 2 9H 2 O), 5 mmol urea and 4 mmol NH 4 F, and 2.4 mmol of ammonium molybdate tetrahydrate ((NH 4 ) 6 Mo 7 o 24 4H 2 O) added to 36 mL of ultrapure water, stirred until completely dissolved, in which Co(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 2 9H 2 O and (NH 4 ) 6 Mo 7 o 24 4H 2 The O molar x / y / z ratio is 1:1:2.4.
[0036] Then, this solution and the nickel foam as the substrate material were transferred into a 50 mL polytetrafluoroethylene-lined stainless steel autoclave, which was sealed and heated at 180 °C for 10 h, and then naturally cooled to room temperature. The product was washed several times with ultrapure water and absolute ethanol, dried under vacuum at 60°C, and finally annealed at 800°C for 2 hours. The resulting product is denoted as Co 1 Fe 1 Mo 2.4 O NSs@NF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com