Keratin nano-flower material loaded with silver phosphate and preparation and application thereof
A technology of keratin and nanoflowers, applied in organic compound/hydride/coordination complex catalysts, color/spectral characteristic measurement, physical/chemical process catalysts, etc., can solve inaccurate physical and chemical properties, complex synthesis process , small amount of synthesis, etc., to achieve the effect of simple and efficient preparation method, good selectivity and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
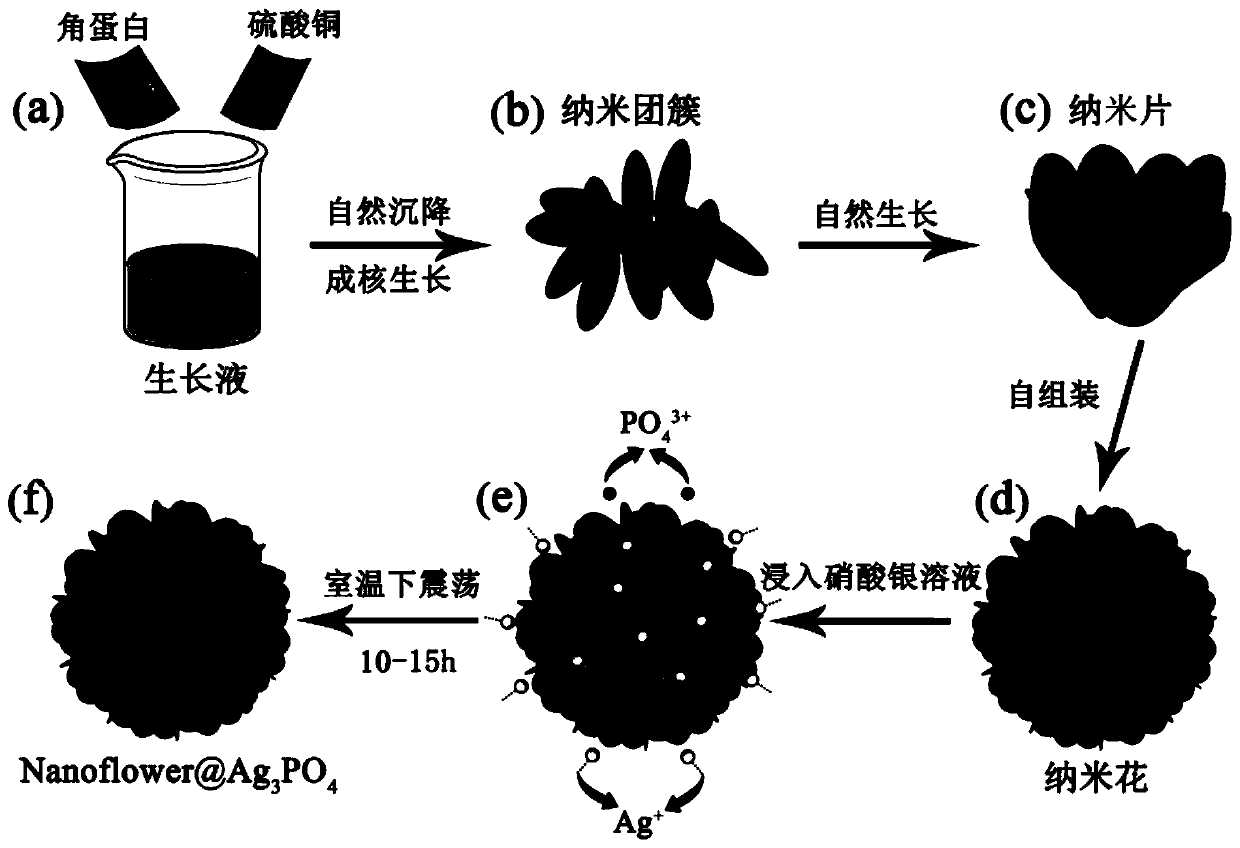
[0039] Such as figure 1 Shown, Keratin-nanoflower@Ag 3 PO 4 The synthesis process is as follows:
[0040] (1) Preparation of Keratin-nanoflower nanoflowers
[0041] ① Accurately weigh 3mg of keratin and dissolve it in PBS phosphate buffer solution (0.1M, pH=7.4);
[0042] ② After mixing evenly, add 200μL (120mM) CuSO to the above solution 4 Solution, rotate and shake the solution, then transfer the centrifuge tube to an electric heating constant temperature water tank at 25°C, and let it settle naturally for 72 hours;
[0043] ③ After centrifuging at 8000rpm for 3 minutes, remove the supernatant, wash the precipitate three times with distilled water, and finally place the obtained precipitate in a vacuum drying oven at 35°C for 12 hours.
[0044] (2) Keratin-nanofower@Ag 3 PO 4 preparation of
[0045] ① Accurately weigh the Keratin-nanoflower (5 mg) synthesized above and disperse it in deionized water;
[0046] ②Weigh again 15mg AgNO 3 dispersed in deionized water; ...
Embodiment 2
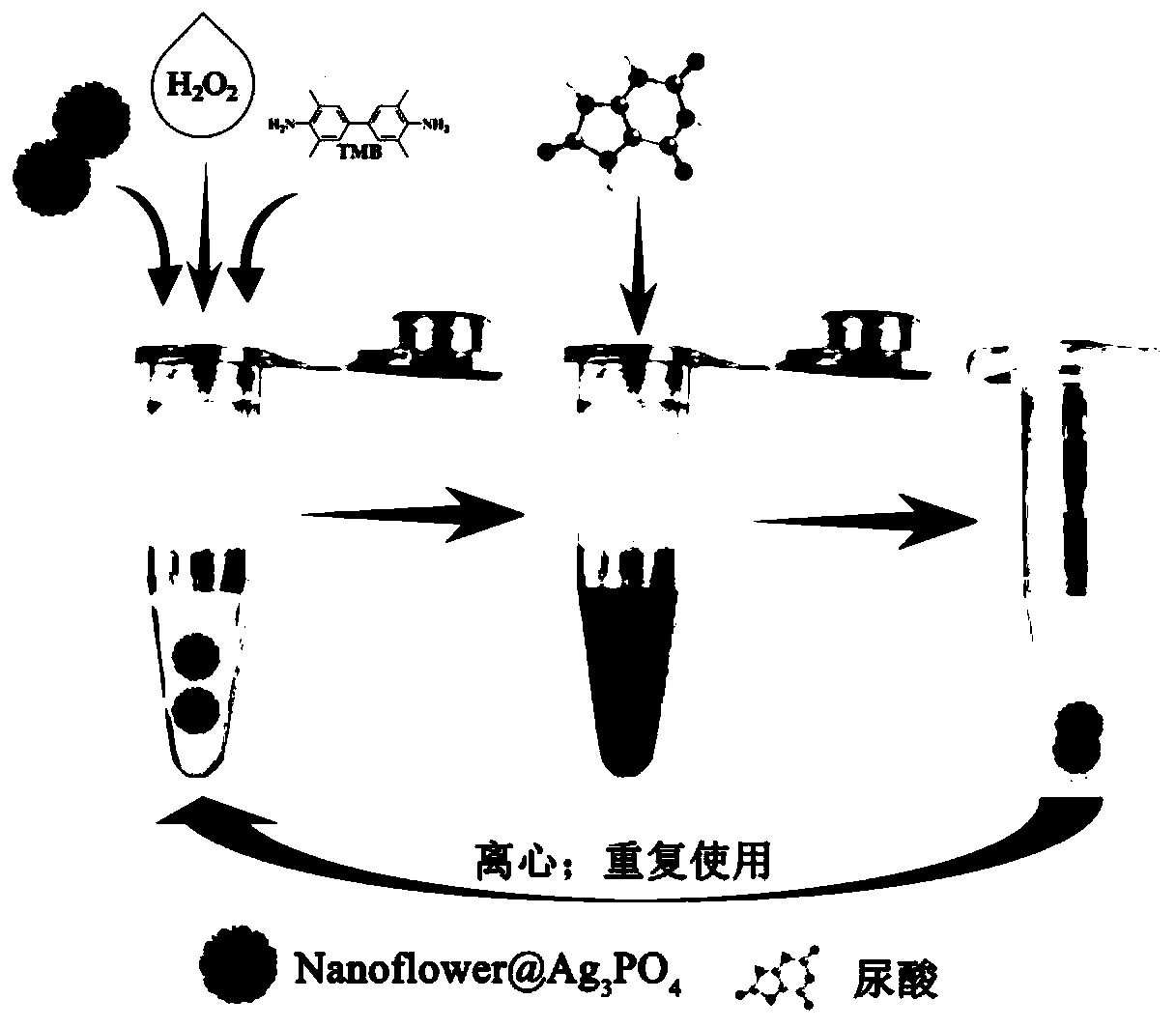
[0053] To Keratin-nanoflower@Ag 3 PO 4 The conditions for catalyzing the oxidation of its substrate by hydrogen peroxide were optimized, among which, Keratin-nanoflower@Ag 3 PO 4 Schematic diagram of colorimetric detection of uric acid figure 2 shown.
[0054] Leverage Keratin-nanoflower@Ag 3 PO 4 The steps of colorimetric detection of uric acid are as follows:
[0055]
Embodiment 21
[0057] Effect of reaction temperature on Keratin-nanoflower@Ag 3 PO 4 Solution Colorimetric Detection of Uric Acid Effect
[0058] (1) Take 360 μL of acetic acid-sodium acetate buffer solution (0.2M, pH=4.00) in a 1.5mL centrifuge tube, and add 20 μL of Keratin-nanoflower@Ag to the centrifuge tube in turn 3 PO 4 (2.5mg / mL), 20 μL hydrogen peroxide aqueous solution (5.0mM), 3,3',5,5'-tetramethylbenzidine (TMB, 2.0mM), mix the above solutions evenly;
[0059] (2) Take part of the mixed solution obtained in step (1) and react in a water bath (20°C, 30°C, 40°C, 50°C, 60°C, 70°C, 80°C) for 8 minutes;
[0060] (3) Keratin-nanoflower@Ag was passed through a centrifuge 3 PO 4 Separated from the reaction solution;
[0061] (4) Measure the ultraviolet absorption spectrum of the above mixed solution with an ultraviolet-visible absorption spectrophotometer.
[0062] Experimental results such as Image 6 As shown, it can be seen from the figure that the absorbance at 652nm increa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com