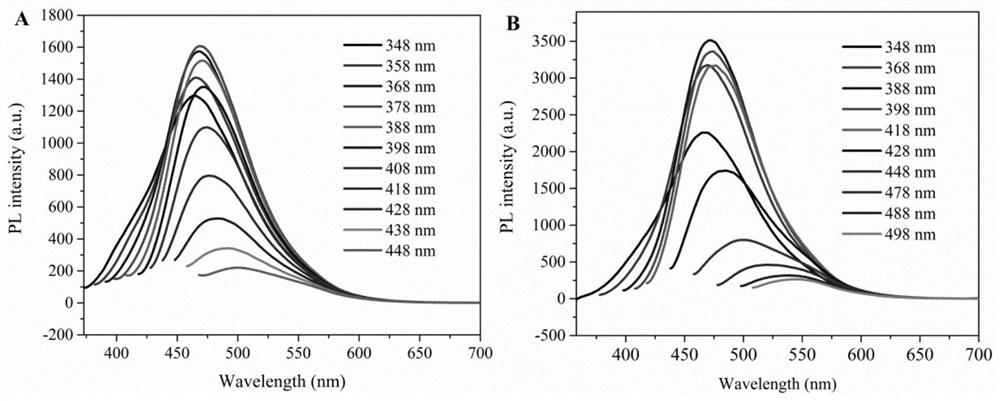
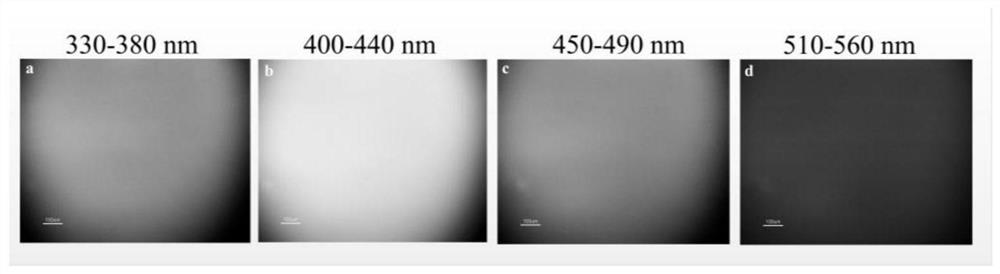
A kind of multicolor fluorescent hyperbranched polyurethane and preparation method thereof
A polyurethane and fluorescence technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of low molecular weight, poor stability, and many by-products of hyperbranched polyurethane, and achieve controllable synthesis process and good stability , low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
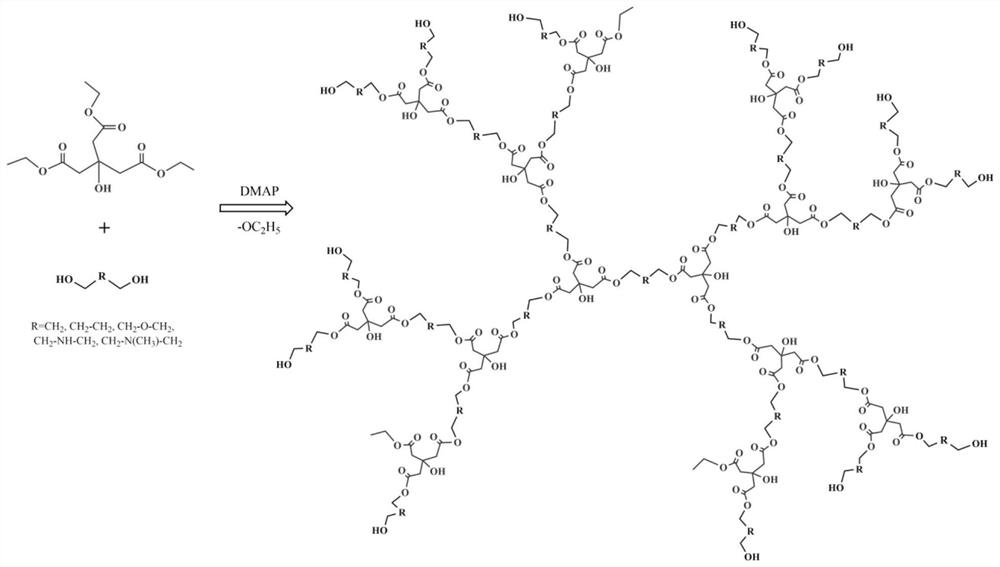
[0026] The preparation method of hyperbranched polyurethane: under the protection of nitrogen, triethyl citrate and diethanolamine are subjected to transesterification reaction at a molar ratio of 1:1. Firstly, the catalyst 4-dimethylaminopyridine (accounting for 0.5-1% of the total mass of reactants) was added, and then reacted at 130° C. for 3 hours. Finally, the product was dissolved in CH 2 Cl 2 , adding ethanol for precipitation, filtration, and vacuum drying to obtain hyperbranched polyurethane.
example 2
[0028] The preparation method of hyperbranched polyurethane: under nitrogen protection, triethyl citrate and diethanolamine are subjected to transesterification at a molar ratio of 1:1.5. Firstly, the catalyst 4-dimethylaminopyridine (accounting for 0.5-1% of the total mass of reactants) was added, and then reacted at 150° C. for 4 hours. Finally, the product was dissolved in CH 2 Cl 2 , adding ethanol for precipitation, filtration, and vacuum drying to obtain hyperbranched polyurethane.
example 3
[0030] Under nitrogen protection, triethyl citrate and diethanolamine were transesterified at a molar ratio of 1:2. First, add catalyst 4-dimethylaminopyridine (accounting for 0.5-1% of the total mass of reactants), and then react at 150° C. for 6 hours. Finally, the product was dissolved in CH 2 Cl 2 , adding ethanol for precipitation, filtration, and vacuum drying to obtain hyperbranched polyurethane.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com